This is a preprint.
Rapid Sterilization of Clinical Apheresis Blood Products using Ultra-High Dose Rate Radiation
- PMID: 39713317
- PMCID: PMC11661200
- DOI: 10.1101/2024.12.14.628469
Rapid Sterilization of Clinical Apheresis Blood Products using Ultra-High Dose Rate Radiation
Update in
-
Rapid Sterilization of Clinical Apheresis Blood Products Using Ultra-High Dose Rate Radiation.Int J Mol Sci. 2025 Mar 7;26(6):2424. doi: 10.3390/ijms26062424. Int J Mol Sci. 2025. PMID: 40141066 Free PMC article.
Abstract
Background and objectives: Apheresis platelets products and plasma are essential for medical interventions, but both still have inherent risks associated with contamination and viral transmission. Platelet products are vulnerable to bacterial contamination due to storage conditions, while plasma requires extensive screening to minimize virus transmission risks. Here we investigate rapid irradiation to sterilizing doses for bacteria and viruses as an innovative pathogen reduction technology.
Materials and methods: We configured a clinical linear accelerator to deliver ultra-high dose rate (6 kGy/min) irradiation to platelet and plasma blood components. Platelet aliquots spiked with 105 CFU of E.coli were irradiated with 0.1-20 kGy, followed by E.coli growth and platelet count assays. COVID Convalescent Plasma (CCP) aliquots were irradiated at a virus-sterilizing dose of 25 kGy and subsequently, RBD-specific antibody binding was assessed.
Results: 1 kGy irradiation of bacteria-spiked platelets reduced E.coli growth by 2.7-log without significant change of platelet count, and 5 kGy or higher produced complete growth suppression. The estimated sterilization (6-log bacterial reduction) dose was 2.3 kGy, corresponding to 31% platelet count reduction. A 25 kGy virus sterilizing dose to CCP produced a 9.2% average drop of RBD-specific IgG binding.
Conclusion: This study shows proof-of-concept of a novel rapid blood sterilization technique using a clinical linear accelerator. Promising platelet counts and CCP antibody binding were maintained at bacteria and virus sterilizing doses, respectively. This represents a potential point-of-care blood product sterilization solution. If additional studies corroborate these findings, this may be a practical method for ensuring blood products safety.
Keywords: Bacteria Spiked Platelets; Blood Products; COVID-19 Convalescent Plasma (CCP); Radiation Based Sterilization; Ultra-High Dose Rate (UHDR).
Conflict of interest statement
CONFLICT OF INTEREST: BWL is a co-founder and board member of TibaRay. BWL is a consultant on a clinical trial steering committee for Beigene and has received lecture honoraria from Mevion. All other authors declare no conflicts of interest.
Figures
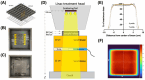
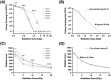
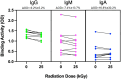
Similar articles
-
Rapid Sterilization of Clinical Apheresis Blood Products Using Ultra-High Dose Rate Radiation.Int J Mol Sci. 2025 Mar 7;26(6):2424. doi: 10.3390/ijms26062424. Int J Mol Sci. 2025. PMID: 40141066 Free PMC article.
-
[Single-donor (apheresis) platelets and pooled whole-blood-derived platelets--significance and assessment of both blood products].Clin Lab. 2014;60(4):S1-39. doi: 10.7754/clin.lab.2014.140210. Clin Lab. 2014. PMID: 24779310 Review. German.
-
The High-cycle Fatigue Life of Cortical Bone Allografts Is Radiation Sterilization Dose-dependent: An In Vitro Study.Clin Orthop Relat Res. 2022 Jun 1;480(6):1208-1219. doi: 10.1097/CORR.0000000000002146. Epub 2022 Feb 17. Clin Orthop Relat Res. 2022. PMID: 35175232 Free PMC article.
-
Effective use of optimized, high-dose (50 kGy) gamma irradiation for pathogen inactivation of human bone allografts.Biomaterials. 2005 May;26(14):2033-42. doi: 10.1016/j.biomaterials.2004.06.028. Biomaterials. 2005. PMID: 15576177
-
Pathogen inactivation of blood components: current status and introduction of an approach using riboflavin as a photosensitizer.Int J Hematol. 2002 Aug;76 Suppl 2:253-7. doi: 10.1007/BF03165125. Int J Hematol. 2002. PMID: 12430933 Review.
References
-
- Godbey EA. Whole Blood Transfusion: Past, Present, and Future. Clin Lab Med. 2021;41:659–67. - PubMed
-
- Steele WR, Dodd RY, Notari EP, Xu M, Nelson D, Kessler DA, et al. Prevalence of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus in United States blood donations, 2015 to 2019: The Transfusion-Transmissible Infections Monitoring System (TTIMS). Transfusion. 2020;60:2327–39. - PubMed
-
- Dodd RY, Crowder LA, Haynes JM, Notari EP, Stramer SL, Steele WR. Screening Blood Donors for HIV, HCV, and HBV at the American Red Cross: 10-Year Trends in Prevalence, Incidence, and Residual Risk, 2007 to 2016. Transfusion Medicine Reviews. 2020;34:81–93. - PubMed
-
- Zou S, Stramer SL, Dodd RY. Donor Testing and Risk: Current Prevalence, Incidence, and Residual Risk of Transfusion-Transmissible Agents in US Allogeneic Donations. Transfusion Medicine Reviews. 2012;26:119–28. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
