This is a preprint.
Environmentally-relevant doses of bisphenol A and S exposure in utero disrupt germ cell programming across generations resolved by single nucleus multi-omics
- PMID: 39713385
- PMCID: PMC11661074
- DOI: 10.1101/2024.12.05.627072
Environmentally-relevant doses of bisphenol A and S exposure in utero disrupt germ cell programming across generations resolved by single nucleus multi-omics
Update in
-
Environmentally-relevant doses of bisphenol A and S exposure in utero disrupt germ cell programming across generations resolved by single nucleus multi-omics.Environ Health Perspect. 2025 Jun 3. doi: 10.1289/EHP16981. Online ahead of print. Environ Health Perspect. 2025. PMID: 40460421
Abstract
Background: Exposure to endocrine-disrupting chemicals (EDCs), such as bisphenol A (BPA), disrupts reproduction across generations. Germ cell epigenetic alterations are proposed to bridge transgenerational reproductive defects resulting from EDCs. Previously, we have shown that prenatal exposure to environmentally relevant doses of BPA or its substitute, BPS, caused transgenerationally maintained reproductive impairments associated with neonatal spermatogonial epigenetic changes in male mice. While epigenetic alterations in germ cells can lead to transgenerational phenotypic variations, the mechanisms sustaining these changes across generations remain unclear.
Objectives: This study aimed to systematically elucidate the mechanism of transgenerational inherence by prenatal BPA and BPS exposure in the murine germline from F1 to F3 generations at both transcriptomic and epigenetic levels.
Methods: BPA or BPS with doses of 0 (vehicle control), 0.5, 50, or 1000 μg/kg/b.w./day was orally administered to pregnant CD-1 females (F0) from gestational day 7 to birth. Sperm counts and motility were examined in F1, F2, and F3 adult males. THY1+ germ cells on postnatal day 6 from F1, F2, and F3 males at a dose of 50 μg/kg/b.w./day were used for analysis by single-nucleus (sn) multi-omics (paired snRNA-seq and snATAC-seq on the same nucleus).
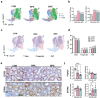
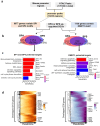
Results: Prenatal exposure to BPA and BPS with 0.5, 50, and 1000 μg/kg/b.w./day reduced sperm counts in mice across F1 to F3 generations. In the F1 neonatal germ cells, ancestral BPA or BPS exposure with 50 μg/kg/b.w./day resulted in increased differentially expressed genes (DEGs) associated with spermatogonial differentiation. It also disrupted the balance between maintaining the undifferentiated and differentiating spermatogonial populations. Differentially accessible peaks (DAPs) by snATAC-seq were primarily located in the promoter regions, with elevated activity of key transcription factors, including SP1, SP4, and DMRT1. Throughout F1-F3 generations, biological processes related to mitosis/meiosis and metabolic pathways were substantially up-regulated in BPA- or BPS-exposed groups. While the quantities of DEGs and DAPs were similar in F1 and F2 spermatogonia, with both showing a significant reduction in F3. Notably, approximately 80% of DAPs in F1 and F2 spermatogonia overlapped with histone post-translational modifications linked to transcription activation, such as H3K4me1/2/3 and H3K27ac. Although BPA exerted more potent effects on gene expression in F1 spermatogonia, BPS induced longer-lasting effects on spermatogonial differentiation across F1 to F3 males. Interestingly, DMRT1 motif activity was persistently elevated across all three generations following ancestral BPA or BPS exposure.
Discussion: Our work provides the first systematic analyses for understanding the transgenerational dynamics of gene expression and chromatin landscape following prenatal exposure to BPA or BPS in neonatal spermatogonia. These results suggest that prenatal exposure to environmentally relevant doses of BPA or BPS alters chromatin accessibility and transcription factor motif activities, consequently contributing to disrupted transcriptional levels in neonatal germ cells, and some are sustained to F3 generations, ultimately leading to the reduction of sperm counts in adults.
Conflict of interest statement
Conflict of interest: The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
