This is a preprint.
Mitochondrial DNA mutations in human oocytes undergo frequency-dependent selection but do not increase with age
- PMID: 39713397
- PMCID: PMC11661235
- DOI: 10.1101/2024.12.09.627454
Mitochondrial DNA mutations in human oocytes undergo frequency-dependent selection but do not increase with age
Abstract
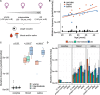
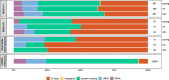
Mitochondria, cellular powerhouses, harbor DNA (mtDNA) inherited from the mothers. MtDNA mutations can cause diseases, yet whether they increase with age in human germline cells-oocytes-remains understudied. Here, using highly accurate duplex sequencing of full-length mtDNA, we detected de novo mutations in single oocytes, blood, and saliva in women between 20 and 42 years of age. We found that, with age, mutations increased in blood and saliva but not in oocytes. In oocytes, mutations with high allele frequencies (≥1%) were less prevalent in coding than non-coding regions, whereas mutations with low allele frequencies (<1%) were more uniformly distributed along mtDNA, suggesting frequency-dependent purifying selection. In somatic tissues, mutations caused elevated amino acid changes in protein-coding regions, suggesting positive or destructive selection. Thus, mtDNA in human oocytes is protected against accumulation of mutations having functional consequences and with aging. These findings are particularly timely as humans tend to reproduce later in life.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Similar articles
-
Allele frequency selection and no age-related increase in human oocyte mitochondrial mutations.Sci Adv. 2025 Aug 8;11(32):eadw4954. doi: 10.1126/sciadv.adw4954. Epub 2025 Aug 6. Sci Adv. 2025. PMID: 40768572 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
The interplay between mitochondrial DNA genotypes, female infertility, ovarian response, and mutagenesis in oocytes.Hum Reprod Open. 2024 Dec 30;2025(1):hoae074. doi: 10.1093/hropen/hoae074. eCollection 2025. Hum Reprod Open. 2024. PMID: 39830711 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Maternal and neonatal outcomes of elective induction of labor.Evid Rep Technol Assess (Full Rep). 2009 Mar;(176):1-257. Evid Rep Technol Assess (Full Rep). 2009. PMID: 19408970 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
