This is a preprint.
Alteration of skin fibroblast steady state contributes to healing outcomes
- PMID: 39713414
- PMCID: PMC11661132
- DOI: 10.1101/2024.12.06.627278
Alteration of skin fibroblast steady state contributes to healing outcomes
Abstract
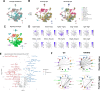
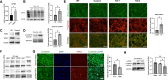
Fibroblasts display complex functions associated with distinct gene expression profiles that influence matrix production and cell communications and the autonomy of tissue development and repair. Thrombospondin-2 (TSP-2), produced by fibroblasts, is a potent angiogenesis inhibitor and negatively associated with tissue repair. Single-cell (sc) sequencing analysis on WT and TSP2KO skin fibroblasts demonstrate distinct cell heterogeneity. Specifically, we found an enrichment of Sox10+ multipotent progenitor cells, identified as Schwann precursor cells, in TSP2KO fibroblasts, while fibrosis-related subpopulations decreased. Immunostaining of tissue and cells validated the increase of this Sox10+ population in KO fibroblasts. Furthermore, in silico analysis suggested enhanced pro-survival signaling, including WNT, TGF-β, and PDGF-β, alongside a reduced BMP4 response. Additionally, the creation of two TSP2KO NIH3T3 cell lines using the CRISPR/Cas9 technique allowed functional and signaling validation in a less complex system. Moreover, KO 3T3 cells exhibited enhanced migration and proliferation, with elevated levels of pro-regenerative molecules including TGF-β3 and Wnt4, and enrichment of nuclear β-catenin. These functional and molecular alterations likely contribute to improved healing and increased neurogenesis in TSP2-deficient wounds. Overall, our findings describe the heterogeneity of dermal fibroblasts and identify pro-regenerative features of TSP2KO fibroblasts.
Keywords: Extracellular matrix; Fibroblasts; Schwann cells; Thrombospondin-2; Transforming growth factor beta; Wnt/β-catenin; tissue state.
Conflict of interest statement
Disclosures We declare that we have no competing financial interests.
Figures





Similar articles
-
Up-regulation of thrombospondin-2 in Akt1-null mice contributes to compromised tissue repair due to abnormalities in fibroblast function.J Biol Chem. 2015 Jan 2;290(1):409-22. doi: 10.1074/jbc.M114.618421. Epub 2014 Nov 11. J Biol Chem. 2015. PMID: 25389299 Free PMC article.
-
Enhanced angiogenesis and reduced contraction in thrombospondin-2-null wounds is associated with increased levels of matrix metalloproteinases-2 and -9, and soluble VEGF.J Histochem Cytochem. 2009 Apr;57(4):301-13. doi: 10.1369/jhc.2008.952689. Epub 2008 Nov 24. J Histochem Cytochem. 2009. PMID: 19029404 Free PMC article.
-
Dedifferentiated Schwann cell-derived TGF-β3 is essential for the neural system to promote wound healing.Theranostics. 2022 Jul 18;12(12):5470-5487. doi: 10.7150/thno.72317. eCollection 2022. Theranostics. 2022. PMID: 35910794 Free PMC article.
-
Upregulation of tumor suppressor protein neurofibromin in normal human wound healing and in vitro evidence for platelet derived growth factor (PDGF) and transforming growth factor-beta1 (TGF-beta1) elicited increase in neurofibromin mRNA steady-state levels in dermal fibroblasts.J Invest Dermatol. 1998 Mar;110(3):232-7. doi: 10.1046/j.1523-1747.1998.00108.x. J Invest Dermatol. 1998. PMID: 9506441 Review.
-
Pathogenesis of scleroderma. Collagen.Rheum Dis Clin North Am. 1996 Nov;22(4):647-74. doi: 10.1016/s0889-857x(05)70294-5. Rheum Dis Clin North Am. 1996. PMID: 8923589 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
