This is a preprint.
Modulation of the JAK2-STAT3 pathway promotes expansion and maturation of human iPSCs-derived myogenic progenitor cells
- PMID: 39713478
- PMCID: PMC11661153
- DOI: 10.1101/2024.12.09.624203
Modulation of the JAK2-STAT3 pathway promotes expansion and maturation of human iPSCs-derived myogenic progenitor cells
Update in
-
Modulation of the JAK2-STAT3 pathway promotes expansion and maturation of human iPSC-derived myogenic progenitor cells.Stem Cell Reports. 2025 Nov 11;20(11):102692. doi: 10.1016/j.stemcr.2025.102692. Epub 2025 Oct 30. Stem Cell Reports. 2025. PMID: 41173008
Abstract
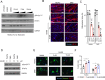
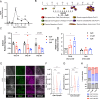
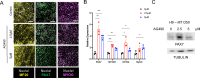
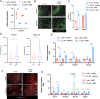
Generation of in vitro induced pluripotent cells (hiPSCs)-derived skeletal muscle progenitor cells (SMPCs) holds great promise for regenerative medicine for skeletal muscle wasting diseases, as for example Duchenne Muscular Dystrophy (DMD). Multiple approaches, involving ectopic expression of key regulatory myogenic genes or small molecules cocktails, have been described by different groups to obtain SMPC towards cell-transplantation in vivo as a therapeutic approach to skeletal muscle diseases. However, hiPSCs-derived SMPC generated using transgene-free protocols are usually obtained in a low amount and resemble a more embryonal/fetal stage of differentiation. Here we demonstrate that modulation of the JAK2/STAT3 signaling pathway during an in vitro skeletal muscle differentiation protocol, increases the yield of PAX7+ and CD54+ SMPCs and drive them to a postnatal maturation stage, in both human ES and patient-derived iPSCs. Importantly, upon removal of the inhibition from the cultures, the obtained SMPCs are able to differentiate into multinucleated myotubes in vitro. These findings reveal that modulation of the JAK2/STAT3 signaling pathway is a potential therapeutic avenue to generate SMPCs in vitro with increase potential for cell-therapy approaches.
Keywords: Duchenne Muscular Dystrophy; STAT3 pathway; hiPSC; myotubes; skeletal muscle progenitor cells.
Conflict of interest statement
Conflict of Interest Statement The authors declare that they have no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
