Exploratory Research for HIF-1α Overexpression Tumor Antigen in the Activation of Dendritic Cells and the Potent Anti-Tumor Immune Response
- PMID: 39713567
- PMCID: PMC11662640
- DOI: 10.2147/CMAR.S482363
Exploratory Research for HIF-1α Overexpression Tumor Antigen in the Activation of Dendritic Cells and the Potent Anti-Tumor Immune Response
Abstract
Background: Tumor-specific antigens play an important role in dendritic cell (DC)-based immunotherapy. The acquisition of tumor-specific antigens, which are essential for DC-based immunotherapy, poses a significant challenge. This study aimed to explore the efficacy of hypoxia inducible factor-1α (HIF-1α) overexpression tumor antigens in DC-based immunotherapy.
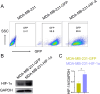
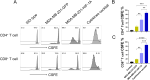
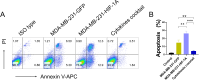
Methods: An HIF-1α over-expression cell line was constructed to prepare HIF-1α overexpression tumor antigens. The expression of CD14, CD40, CD80, CD86, and HLA-DR on the surface of dendritic cells derived from monocytes was assessed using flow cytometry after stimulation with tumor antigens enriched in HIF-1α. T cell proliferation was analyzed by CFSE division following incubation with mature DCs. The apoptotic tumor cells were detected through annexin V/PI staining following coculture with dendritic cells (DCs) stimulated by HIF-1α enriched antigens. The detection of damage-associated molecular pattern molecules (DAMPs) HMGB1 and calreticulin (CALR) was performed using Western blotting.
Results: The results demonstrated that HIF-1α-enriched tumor antigens significantly upregulated the expression of CD40, CD80, CD86, and HLA-DR in DCs compared to normal tumor antigens. Furthermore, co-incubation with HIF-1α-enriched tumor antigen-activated DCs enhanced T cell proliferation and stimulated the T cell-mediated cytotoxicity. Notably, the expression of DAMPs, such as HMGB1 and CALR, was elevated in HIF-1α-enriched tumor antigens.
Conclusion: Our findings demonstrate that tumor antigens enriched with HIF-1α may encompass tumor-specific antigens capable of stimulating DC activation, thereby enhancing T cell proliferation and cytotoxicity. These results provide support for the further advancement of HIF-1α enriched tumor antigens in preclinical and clinical investigations pertaining to tumor treatment.
Keywords: HIF-1α; HMGB1; T cell activation; calreticulin; dendritic cells.
© 2024 Zhao et al.
Conflict of interest statement
The authors declare that they have no competing interests in this work.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

