Charging and Discharging of Poly(m-aminophenylboronic Acid) Doped with Phytic Acid for Enzyme-Free Real-Time Monitoring of Human Sweat Lactate
- PMID: 39713628
- PMCID: PMC11656369
- DOI: 10.1021/acsomega.4c06671
Charging and Discharging of Poly(m-aminophenylboronic Acid) Doped with Phytic Acid for Enzyme-Free Real-Time Monitoring of Human Sweat Lactate
Abstract
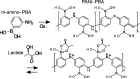
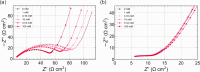
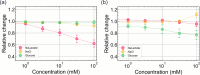
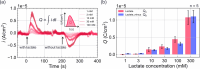
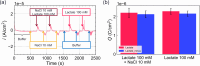
In this study, we realized a real-time and enzyme-free measurement of lactate in sweat in the same way as an enzyme-based amperometric method. A conductive polymer, which is based on polyaniline (PANI), was strongly coated on a glassy carbon electrode as a poly m-aminophenylboronic acid (PANI-PBA) membrane by drop-casting, which is a convenient method, owing to adhesive phytic acid (PA) molecules with negative charges included as a dopant. This polymer membrane had a functional structure with PBA in the PANI main chain, which expectedly induced electrical charges upon diol binding to lactate, owing to the formation of deprotonated boronate esters with negative charges. This indicates that PBA served as the self-dopant and as the site of binding to lactate. On the basis of the fundamental electrochemical characteristics such as the membrane resistance, the change in the current density of the PA-doped PANI-PBA electrode was quantitatively monitored with the change in lactate concentration from 1 to 300 mM under acidic conditions in real time, considering pH and interfering substances in sweat. Moreover, the sweat lactate concentration was determined to be ca. 60 mM using the PA-doped PANI-PBA electrode in a microfluidic system in measurements using sweat samples collected during exercise load. A change in current density induced a change in the density of charges in the capacitive PA-doped PANI-PBA membrane. This means that the detection mechanism for the change in the lactate concentration in sweat was based on repeated charging and discharging in the PA-doped PANI-PBA electrode.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
LinkOut - more resources
Full Text Sources
