Synthesis of N-Heteroaryl C-Glycosides and Polyhydroxylated Alkanes with Diaryl Groups from Unprotected Sugars
- PMID: 39713645
- PMCID: PMC11656392
- DOI: 10.1021/acsomega.4c07671
Synthesis of N-Heteroaryl C-Glycosides and Polyhydroxylated Alkanes with Diaryl Groups from Unprotected Sugars
Abstract
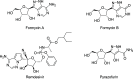
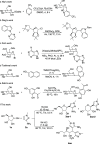
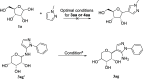
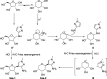
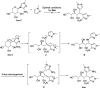
HCl-catalyzed C-glycosylation was described herein for the convenient preparation of N-heteroaryl C-glycosides and polyhydroxylated alkanes with diaryl groups using hetereoaryl amines and unprotected sugars as starting materials. The reaction temperature and the amounts of aryl amines and HCl had significant effects on reactions. The method provided a highly efficient and environmentally friendly route for constructing C-glycosides at low cost.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
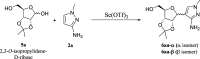
Similar articles
-
One-pot synthesis of novel iminosugar C-nucleosides based on the N-aryl iminium-ion.Carbohydr Res. 2025 Oct;556:109594. doi: 10.1016/j.carres.2025.109594. Epub 2025 Jul 5. Carbohydr Res. 2025. PMID: 40644819
-
FeCl3-Mediated One-Pot Domino Reactions for the Synthesis of 9-Aryl/9-Arylethynyl-2,3,4,9-tetrahydro-1H-xanthen-1-ones from Propargylic Amines/Diaryl Amines and 1,3-Cyclohexanediones.J Org Chem. 2016 Mar 4;81(5):2062-9. doi: 10.1021/acs.joc.6b00001. Epub 2016 Feb 22. J Org Chem. 2016. PMID: 26878148
-
Rhodium-catalyzed transformation of heteroaryl aryl ethers into heteroaryl fluorides.Chem Commun (Camb). 2016 Sep 15;52(76):11390-11393. doi: 10.1039/c6cc05400e. Chem Commun (Camb). 2016. PMID: 27709178
-
O-Glycosylation methods in the total synthesis of complex natural glycosides.Nat Prod Rep. 2015 Sep;32(9):1331-55. doi: 10.1039/c5np00033e. Nat Prod Rep. 2015. PMID: 26067865 Review.
-
Novel glycosylation methods and their application to natural products synthesis.Carbohydr Res. 2006 Jul 24;341(10):1282-97. doi: 10.1016/j.carres.2006.03.012. Epub 2006 Apr 21. Carbohydr Res. 2006. PMID: 16630601 Review.
References
LinkOut - more resources
Full Text Sources
