In Vitro and In Vivo Comparison of Random versus Site-Specific Conjugation of Bifunctional Chelating Agents to the CD33-Binding Antibody for Use in Alpha- and Beta-Radioimmunotherapy
- PMID: 39713652
- PMCID: PMC11656351
- DOI: 10.1021/acsomega.4c09450
In Vitro and In Vivo Comparison of Random versus Site-Specific Conjugation of Bifunctional Chelating Agents to the CD33-Binding Antibody for Use in Alpha- and Beta-Radioimmunotherapy
Erratum in
-
Correction to "In Vitro and In Vivo Comparison of Random versus Site-Specific Conjugation of Bifunctional Chelating Agents to the CD33-Binding Antibody for Use in Alpha- and Beta-Radioimmunotherapy".ACS Omega. 2025 Jul 10;10(28):31203. doi: 10.1021/acsomega.5c06157. eCollection 2025 Jul 22. ACS Omega. 2025. PMID: 40727830 Free PMC article.
Abstract
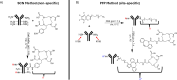
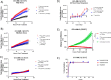
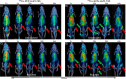
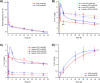
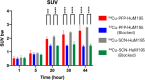
Radiometal chelator conjugation is a cornerstone of radioimmunotherapy (RIT). Continued interest in selective placement of chelators remains an active topic of discussion in the field. With several simple site-specific methods being recently reported, it was of interest to investigate the benefits and potential drawbacks of the site-specific method with a full comparison to a more typical random conjugation method that is currently utilized in clinical applications. In this study, the conjugation methods were evaluated side by side to determine the utility of both methods using commercially available random and site-specific conjugation reagents by performing antigen binding; radiolabeling with 64Cu, 177Lu, and 225Ac radioisotopes; antibody-conjugate stability, cytotoxicity, in vivo distribution, pharmacokinetics analyses, and dosimetry to gather a whole data set for preclinical investigation. Evaluation revealed that both methods performed similarly during most experiments with the site-specific method, resulting in higher binding capacity of the antibody conjugate via flow cytometry. Radiolabeling was not significantly different between two methods, while stability showed that the site-specifically conjugated antibody was somewhat more stable at 37 °C in human serum over 1 week. In vitro experiments demonstrated less cell killing with the random conjugation method, while in vivo experiments showed no statistical differences in tumor uptake between conjugation methods. Dosimetry calculations were performed using the acquired PET/CT data and showed that apart from the liver, there was no significant difference in radiation doses delivered by either antibody conjugate. These results demonstrate that both methods are viable for future work, while the site-specific method offers several potential advantages and, in some cases, improved efficacy.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- El Alaoui M.; Sivado E.; Jallas A.-C.; Mebarki L.; Dyson M. R.; Perrez F.; Valsesia-Wittmann S.; El Alaoui S. Antibody and antibody fragments site-specific conjugation using new Q-tag substrate of bacterial transglutaminase. Cell Death Discov. 2024, 10 (1), 79. 10.1038/s41420-024-01845-3. - DOI - PMC - PubMed
-
- Sarrett S. M.; Rodriguez C.; Rymarczyk G.; Hosny M. M.; Keinänen O.; Delaney S.; Thau S.; Krantz B. A.; Zeglis B. M. Lysine-Directed Site-Selective Bioconjugation for the Creation of Radioimmunoconjugates. Bioconjugate Chem. 2022, 33 (9), 1750–1760. 10.1021/acs.bioconjchem.2c00354. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
