Cooperative Molecular Interaction-Based Highly Efficient Capturing of Ultrashort- and Short-Chain Emerging Per- and Polyfluoroalkyl Substances Using Multifunctional Nanoadsorbents
- PMID: 39713664
- PMCID: PMC11656356
- DOI: 10.1021/acsomega.4c07159
Cooperative Molecular Interaction-Based Highly Efficient Capturing of Ultrashort- and Short-Chain Emerging Per- and Polyfluoroalkyl Substances Using Multifunctional Nanoadsorbents
Abstract
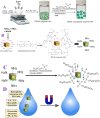
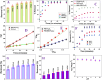
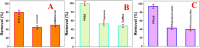
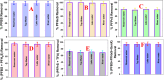
The short-chain (C4 to C7) and ultrashort-chain (C3 to C2) per- and polyfluoroalkyl substances (PFAS) are bioaccumulative, carcinogenic to humans, and harder to remove using current technologies, which are often detected in drinking and environmental water samples. Herein, we report the development of nonafluorobutanesulfonyl (NFBS) and polyethylene-imine (PEI)-conjugated Fe3O4 magnetic nanoparticle-based magnetic nanoadsorbents and demonstrated that the novel adsorbent has the capability for highly efficient removal of six different short- and ultrashort-chain PFAS from drinking and environmental water samples. Reported experimental data indicates that by capitalizing the cooperative hydrophobic, fluorophilic, and electrostatic interaction processes, NFBS-PEI-conjugated magnetic nanoadsorbents can remove ∼100% short-chain perfluorobutanesulfonic acid within 30 min from the water sample with a maximum absorption capacity q m of ∼234 mg g-1. Furthermore, to show how cooperative interactions are necessary for effective capturing of ultrashort and short PFAS, a comparative study has been performed using PEI-attached magnetic nanoadsorbents without NFBS and acid-functionalized magnetic nanoadsorbents without PEI and NFBS. Reported data show that the ultrashort-chain perfluoropropanesulfonic acid capture efficiency is the highest for the NFBS-PEI-attached nanoadsorbent (q m ∼ 187 mg g-1) in comparison to the PEI-attached nanoadsorbent (q m ∼ 119 mg g-1) or carboxylic acid-attached nanoadsorbent (q m ∼ 52 mg g-1). In addition, the role of cooperative molecular interactions in highly efficient removal of ultrashort-chain PFAS has been analyzed in detail. Moreover, reported data demonstrate that nanoadsorbents can be used for effective removal of short-chain PFAS (<92%) and ultrashort-chain PFAS (<70%) simultaneously from reservoir, lake, tape, and river water samples within 30 min, which shows the potential of nanoadsorbents for real-life PFAS remediation.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- United States Environmental Protection Agency . Technical Fact Sheet: Drinking Water Health Advisories for Four PFAS (PFOA, PFOS, GenX Chemicals and PFBS). https://www.epa.gov/system/files/documents/2022-06/drinking-water-ha-pfa... (Accessed July 28, 2024).
-
- Lim X. Can the World Leave ‘Forever Chemicals’ Behind?. Nature 2023, 620, 24–27. 10.1038/d41586-023-02444-5. - DOI
-
- Neuwald I. J.; Hübner D.; Wiegand H. L.; Valkov V.; Borchers U.; Nödler K.; Scheurer M.; Hale S. E.; Arp H. P. H.; Zahn D. Ultra-Short-Chain PFASs in the Sources of German Drinking Water: Prevalent, Overlooked, Difficult to Remove, and Unregulated. Environ. Sci. Technol. 2022, 56 (10), 6380–6390. 10.1021/acs.est.1c07949. - DOI - PubMed
-
- Evich M. G.; Davis M. J. B.; McCord J. P.; Acrey B.; Awkerman J. A.; Knappe D. R. U.; Lindstrom A. B.; Speth T. F.; Tebes-Stevens C.; Strynar M. J.; Wang Z.; Weber E. J.; Henderson W. M.; Washington J. W. Per- and polyfluoroalkyl substances in the environment. Science 2022, 375, eabg9065 10.1126/science.abg9065. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
