Effect of Gold Nanoparticles on the Conformation of Bovine Serum Albumin: Insights from CD Spectroscopic Analysis and Molecular Dynamics Simulations
- PMID: 39713703
- PMCID: PMC11656231
- DOI: 10.1021/acsomega.4c06409
Effect of Gold Nanoparticles on the Conformation of Bovine Serum Albumin: Insights from CD Spectroscopic Analysis and Molecular Dynamics Simulations
Abstract
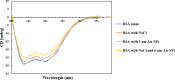
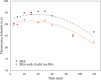
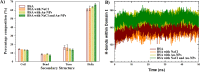
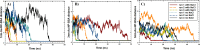
With the development of nanotechnology, there is growing interest in using nanoparticles (NPs) for biomedical applications, such as diagnostics, drug delivery, imaging, and nanomedicine. The protein's structural stability plays a pivotal role in its functionality, and any alteration in this structure can have significant implications, including disease progression. Herein, we performed a combined experimental and computational study of the effect of gold NPs with a diameter of 5 nm (5 nm Au-NPs) on the structural stability of bovine serum albumin (BSA) protein in the absence and presence of NaCl salt. Circular dichroism spectroscopy showed a loss in the secondary structure of BSA due to the synergistic effect of Au-NPs and NaCl, and Thioflavin T fluorescence assays showed suppressed β-sheet formation in the presence of Au-NPs in PBS, emphasizing the intricate interplay between NPs and physiological conditions. Additionally, molecular dynamics (MD) simulations revealed that 5 nm Au-NP induced changes in the secondary structure of the BSA monomer in the presence of NaCl, highlighting the initial binding mechanism between BSA and Au-NP. Furthermore, MD simulations explored the effect of smaller Au-NP (3 nm) and nanocluster (Au-NC with the size of 1 nm) on the binding sites of the BSA monomer. Although the formation of stable BSA-Au conjugates was revealed in the presence of NPs of different sizes, no specific protein binding sites were observed. Moreover, due to its small size, 1 nm Au-NC decreased helical content and hydrogen bonds in the BSA monomer, promoting protein unfolding more significantly. In summary, this combined experimental and computational study provides comprehensive insights into the interactions among Au nanosized substances, BSA, and physiological conditions that are essential for developing tailored nanomaterials with enhanced biocompatibility and efficacy.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Elucidating the influence of gold nanoparticles on the binding of salvianolic acid B and rosmarinic acid to bovine serum albumin.PLoS One. 2015 Apr 10;10(4):e0118274. doi: 10.1371/journal.pone.0118274. eCollection 2015. PLoS One. 2015. PMID: 25861047 Free PMC article.
-
Drug Binding to Partially Unfolded Serum Albumin: Insights into Nonsteroidal Anti-Inflammatory Drug Naproxen-BSA Interactions from Spectroscopic and MD Simulation Studies.J Phys Chem B. 2024 Oct 3;128(39):9327-9340. doi: 10.1021/acs.jpcb.4c03901. Epub 2024 Sep 24. J Phys Chem B. 2024. PMID: 39316707
-
Probing multifunctional azure B conjugated gold nanoparticles with serum protein binding properties for trimodal photothermal, photodynamic, and chemo therapy: Biophysical and photophysical investigations.Biomater Adv. 2022 Mar;134:112678. doi: 10.1016/j.msec.2022.112678. Epub 2022 Jan 24. Biomater Adv. 2022. PMID: 35606220
-
Multispectroscopic and bioimaging approach for the interaction of rhodamine 6G capped gold nanoparticles with bovine serum albumin.J Photochem Photobiol B. 2018 Jun;183:374-384. doi: 10.1016/j.jphotobiol.2018.05.005. Epub 2018 May 7. J Photochem Photobiol B. 2018. PMID: 29763760
-
Nanoparticle-cell interactions: molecular structure of the protein corona and cellular outcomes.Acc Chem Res. 2014 Aug 19;47(8):2651-9. doi: 10.1021/ar500190q. Epub 2014 Jul 11. Acc Chem Res. 2014. PMID: 25014679 Free PMC article.
References
-
- Bashiri G.; Padilla M. S.; Swingle K. L.; Shepherd S. J.; Mitchell M. J.; Wang K. Nanoparticle protein corona: from structure and function to therapeutic targeting. Lab Chip 2023, 23 (6), 1432–1466. 10.1039/D2LC00799A. - DOI - PMC - PubMed
- Ahmad A.; Georgiou P. G.; Pancaro A.; Hasan M.; Nelissen I.; Gibson M. I. Polymer-tethered glycosylated gold nanoparticles recruit sialylated glycoproteins into their protein corona, leading to off-target lectin binding. Nanoscale 2022, 14 (36), 13261–13273. 10.1039/D2NR01818G. - DOI - PMC - PubMed
- Wang G.; Yan C.; Gao S.; Liu Y. Surface chemistry of gold nanoparticles determines interactions with bovine serum albumin. Materials Science and Engineering: C 2019, 103, 10985610.1016/j.msec.2019.109856. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
