An in vivo evaluation of the safety and efficacy of using decellularized bovine parietal peritoneum membranes as dural substitutes
- PMID: 39713807
- PMCID: PMC11659263
- DOI: 10.3389/fsurg.2024.1432029
An in vivo evaluation of the safety and efficacy of using decellularized bovine parietal peritoneum membranes as dural substitutes
Abstract
Purpose: The reconstruction of dura matter is a challenging problem for neurosurgeons. A number of materials for dural reconstruction have recently been developed, but some of them have poor biocompatibility, poor mechanical properties, and adverse effects. Bovine parietal peritoneum is a promising natural material for regenerative medicine and reconstructive surgery. In this study, we conducted an in vivo evaluation of the safety and efficacy of using decellularized bovine peritoneum membranes (BPMs) as natural dural substitutes in a rabbit model.
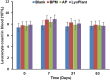
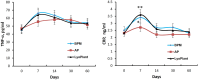
Methods: The dural defects in mature New Zealand rabbits were studied. A BPM was sutured on the dural defect area of each animal. Autologous periosteum and collagen membranes (Lyoplant®) were used to facilitate a comparison with the BPMs. ELISA, histomorphological analysis, and hematological analysis were carried out to examine the safety and efficacy of using BPMs as dural substitutes.
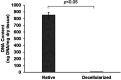
Results: Our results showed that the BPMs demonstrated a deterioration rate that is suitable for gathering newly formed meningothelial tissue. The thickness and density of BPM fibers prevents resorption in the first few days after use as a plastic material, and the regeneration of the dura mater does not occur at an accelerated pace, meaning that the gradual formation of fibrous tissue prevents adhesion to the brain surface. It was observed that the BPM can integrate with the adjacent tissue to repair dural defects. Moreover, the transplantation of BPMs did not cause significant adverse effects or immunological responses, indicating the safety and good biocompatibility of the BPM.
Conclusion: Thus, our in vivo study in a rabbit model showed that decellularized BPMs may represent a biocompatible natural material that can be used in cases requiring dura matter repair without significant adverse effects.
Keywords: bovine parietal peritoneum; dura matter; dural substitute; duraplasty; regeneration.
© 2024 Doskaliyev, Ogay, Mussabekov, Satov, Zhetpisbayev, Mustafin, Bobrova, Auezova and Akshulakov.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



 and blood vessels
and blood vessels  . (A–C,E–G,I–K) H&E staining; (D,H,L) Masson's trichrome (TRI) staining.
. (A–C,E–G,I–K) H&E staining; (D,H,L) Masson's trichrome (TRI) staining.
 and blood vessels
and blood vessels  . (A–C,E–G,I–K) H&E staining; (D,H,L) Masson's trichrome (TRI) staining.
. (A–C,E–G,I–K) H&E staining; (D,H,L) Masson's trichrome (TRI) staining.
 and blood vessels
and blood vessels  . (A–C,E–G,I–K) H&E staining; (D,H,L) Masson's trichrome (TRI) staining.
. (A–C,E–G,I–K) H&E staining; (D,H,L) Masson's trichrome (TRI) staining.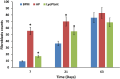
References
-
- Paek SH, Audu PB, Sperling MR, Cho J, Andrews DW. Reevaluation of surgery for the treatment of brain metastases: review of 208 patients with single or multiple brain metastases treated at one institution with modern neurosurgical techniques. Neurosurgery. (2005) 56(5):1021–34. 10.1227/01.NEU.0000158321.90608.BE - DOI - PubMed
LinkOut - more resources
Full Text Sources

