Microglia internalize tau monomers and fibrils using distinct receptors but similar mechanisms
- PMID: 39713861
- PMCID: PMC11848386
- DOI: 10.1002/alz.14418
Microglia internalize tau monomers and fibrils using distinct receptors but similar mechanisms
Abstract
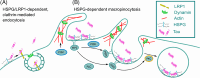
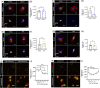
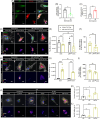
Introduction: Alzheimer's disease (AD) and other tauopathies are characterized by intracellular aggregates of microtubule-associated protein tau that are actively released and promote proteopathic spread. Microglia engulf pathological proteins, but how they endocytose tau is unknown.
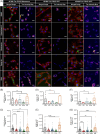
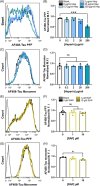
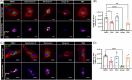
Methods: We measured endocytosis of different tau species by microglia after pharmacological modulation of macropinocytosis or clathrin-mediated endocytosis (CME) or antagonism/genetic depletion of known tau receptors heparan-sulfate proteoglycans (HSPGs) and low-density lipoprotein receptor-related protein 1 (LRP1).
Results: Dynamin inhibition decreased microglial endocytosis of all tested tau species. Meanwhile, HSPG antagonism blocked only fibril uptake, and LRP1 antagonism or genetic depletion inconsistently inhibited the endocytosis of fibrils and monomers. Cre recombinase robustly enhanced tau uptake with partial selectivity for fibrils.
Discussion: These data show that microglia take up both tau monomers and aggregates via a dynamin-dependent form of endocytosis (eg, CME) but may differ in using HSPGs for entry depending on species.
Highlights: Microglial endocytosis of tau monomers and fibrils is dynamin-dependent. HSPG antagonism blocks microglial uptake of tau fibrils but not monomers. LRP1 antagonism or knockdown inconsistently inhibits tau uptake. TAT-Cre stimulates semi-selective uptake of fibrils over monomers.
Keywords: Alzheimer's disease; endocytosis; microglia; neuroinflammation; tau; tauopathy.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Ariel Louwrier is the founder of StressMarq Biosciences, who provided recombinant tau for use in this study, and Jacob A. McPhail was employed by StressMarq Biosciences during the time the work was performed. The authors declare that they have no other competing interests. Author disclosures are available in the Supporting Information.
Figures


References
-
- Santa‐Maria I, Varghese M, Ksiȩzak‐Reding H, Dzhun A, Wang J, Pasinetti GM. Paired helical filaments from Alzheimer disease brain induce intracellular accumulation of tau protein in aggresomes. J Biol Chem. 2012;287(24):20522‐20533. doi: 10.1074/JBC.M111.323279/ATTACHMENT/983165C9-D389-4139-8453-C99817274557/MMC1.PDF - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- R21 AG072423/AG/NIA NIH HHS/United States
- P30CA054174/CA/NCI NIH HHS/United States
- Max and Minnie Tomerlin Voelcker Fund
- Dan & Kelley McNamara Alzheimer's Research Fund
- RP210126/Cancer Prevention and Research Institute of Texas
- K01AG066747/NH/NIH HHS/United States
- P30 AG066546/AG/NIA NIH HHS/United States
- 679-2021-12/CurePSP foundation
- IK2BX003240/US Department of Veteran Affairs
- P30 CA054174/CA/NCI NIH HHS/United States
- S10 OD030432/OD/NIH HHS/United States
- NS11552/NH/NIH HHS/United States
- K01 AG066747/AG/NIA NIH HHS/United States
- T32 NS082145/NS/NINDS NIH HHS/United States
- P30AG066546/NH/NIH HHS/United States
- NS082145/NH/NIH HHS/United States
- R01 AG085531/AG/NIA NIH HHS/United States
- AARG-21-846012/ALZ/Alzheimer's Association/United States
- R01AG085531-A1/NH/NIH HHS/United States
- R21AG072423/NH/NIH HHS/United States
- S10OD030432/NH/NIH HHS/United States
- R25 NS115552/NS/NINDS NIH HHS/United States
- IK2 BX003240/BX/BLRD VA/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

