Structured Polymers Enable the Sustained Delivery of Glucocorticoids within the Intra-Articular Space
- PMID: 39713898
- PMCID: PMC11804841
- DOI: 10.1002/adhm.202403000
Structured Polymers Enable the Sustained Delivery of Glucocorticoids within the Intra-Articular Space
Abstract
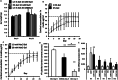
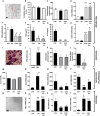
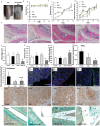
Intra-articular glucocorticoid injections are effective in controlling inflammation and pain in arthritides but restricted by short duration of action and risk of joint degeneration. Controlled drug release using biocompatible hydrogels offers a unique solution, but limitations of in situ gelation restrict their application. Gellan sheared hydrogels (GSHs) retain the advantages of hydrogels, however their unique microstructures lend themselves to intra-articular application - capable of shear thinning under force but restructuring at rest to enhance residence. This study examined GSHs for extended intra-articular glucocorticoid delivery of prednisolone (10 mg mL-1); demonstrating links between material mechanics, steroid release, and preclinical assessment of efficacy in synoviocyte culture and transgenic(TNF)197Gkl (TNFtg) murine model of arthritis. GSHs demonstrated sustained release, with typical Fickian profiles over 18 days. Moreover, systems showed good stability under extended culture, with inherent cell-compatibility and suppression of inflammatory synoviocyte activation. In TNFtg animals, GSHs suppressed synovitis (70.08%, p < 0.05), pannus formation (45.01%, p < 0.05), and increased articular cartilage (82.23%, p < 0.05) relative to vehicle controls. The extended profile of steroid release from injectable GSH formulations holds promise in the treatment and management of inflammatory arthritides such as rheumatoid and osteoarthritis, representing a step-change in intra-articular drug delivery to suppress long-term joint inflammation.
Keywords: arthritis; gellan; glucocorticoid; intra‐articular; sheared hydrogel.
© 2024 The Author(s). Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
Both L.M. Grover and R.J.A. Moakes are inventors of a patent describing the production of sheared gels and are founders in a company seeking to commercialize the technology (Healome Therapeutics Ltd).
Figures


References
-
- Deyle G. D., Allen C. S., Allison S. C., Gill N. W., Hando B. R., Petersen E. J., Dusenberry D. I., Rhon D. I., N. Engl. J. Med. 2020, 382, 1420. - PubMed
-
- Hajialilo M., Ghorbanihaghjo A., Valaee L., Kolahi S., Rashtchizadeh N., Amirkhiz M. B., Malekmahdavi I., Khabbazi A., Clin. Rheumatol. 2016, 35, 2887. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

