Alzheimer's Association clinical practice guideline for the Diagnostic Evaluation, Testing, Counseling, and Disclosure of Suspected Alzheimer's Disease and Related Disorders (DETeCD-ADRD): Executive summary of recommendations for primary care
- PMID: 39713942
- PMCID: PMC12173843
- DOI: 10.1002/alz.14333
Alzheimer's Association clinical practice guideline for the Diagnostic Evaluation, Testing, Counseling, and Disclosure of Suspected Alzheimer's Disease and Related Disorders (DETeCD-ADRD): Executive summary of recommendations for primary care
Abstract
US clinical practice guidelines for the diagnostic evaluation of cognitive impairment due to Alzheimer's disease (AD) or AD and related dementias (ADRD) are decades old and aimed at specialists. This evidence-based guideline was developed to empower all-including primary care-clinicians to implement a structured approach for evaluating a patient with symptoms that may represent clinical AD/ADRD. Through a modified-Delphi approach and guideline-development process (7374 publications were reviewed; 133 met inclusion criteria) an expert workgroup developed recommendations as steps in a patient-centered evaluation process. This summary focuses on recommendations, appropriate for any practice setting, forming core elements of a high-quality, evidence-supported evaluation process aimed at characterizing, diagnosing, and disclosing the patient's cognitive functional status, cognitive-behavioral syndrome, and likely underlying brain disease so that optimal care plans to maximize patient/care partner dyad quality of life can be developed; a companion article summarizes specialist recommendations. If clinicians use this guideline and health-care systems provide adequate resources, outcomes should improve in most patients in most practice settings. Highlights US clinical practice guidelines for the diagnostic evaluation of cognitive impairment due to Alzheimer's disease (AD) or AD and related dementias (ADRD) are decades old and aimed at specialists. This evidence-based guideline was developed to empower all-including primary care-clinicians to implement a structured approach for evaluating a patient with symptoms that may represent clinical AD/ADRD. This summary focuses on recommendations, appropriate for any practice setting, forming core elements of a high-quality, evidence-supported evaluation process aimed at characterizing, diagnosing, and disclosing the patient's cognitive functional status, cognitive-behavioral syndrome, and likely underlying brain disease so that optimal care plans to maximize patient/care partner dyad quality of life can be developed; a companion article summarizes specialist recommendations. If clinicians use this guideline and health-care systems provide adequate resources, outcomes should improve in most patients in most practice settings.
Keywords: Alzheimer's disease; Lewy body dementia; cerebrospinal fluid; dementia; diagnosis; frontotemporal dementia; magnetic resonance imaging; mild cognitive impairment; molecular biomarkers; positron emission tomography; vascular cognitive impairment.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Bradford C. Dickerson: consulting for Acadia, Alector, Arkuda, Biogen, Eisai, Med Learning Group, Quanterix; on DSMB for Lilly, Merck; royalties from Cambridge University Press, Elsevier, Oxford University Press, Up To Date. Alireza Atri: consulting for Acadia, AriBio, AZ Therapies, Biogen, Eisai, JOMDD, Lundbeck, Life Molecular Imaging, Merck, ONO, Prothena, Roche/Genentech, Novo Nordisk, Qynapse, Vaxxinity; royalties from Oxford University Press. Carolyn Clevenger: none. Jason Karlawish: on a DSMB for Linus Health. David Knopman: on a DSMB for DIAN TU. Pei‐Jung Lin: consulting for Lilly. Mary Norman: none. Chiadi Onyike: consulting for Acadia Pharmaceuticals, Reata Pharmaceuticals, Otsuka Pharmaceutical, Eisai Pharmaceutical, Lykos Therapeutics, Zevra Therapeutics. Mary Sano: consulting for Eisai, NovoNordisk, Otsuka Lundbeck. Susan Scanland: employee of Dementia Connection, LLC; consulting for Axsome, BioXcel, Eisai, Genentech, Lundbeck, Otsuka. Maria Carrillo: employee of Alzheimer's Association. Author disclosures are available in the supporting information.
Figures


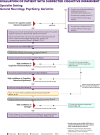
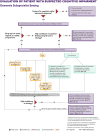
References
-
- 2023 Alzheimer's disease facts and figures. Alzheimers Dement. 2023;19:1598‐1695. - PubMed
-
- 2020 Alzheimer's disease facts and figures. Alzheimers Dementia. 2020;16:391‐460. - PubMed
-
- 2018 Alzheimer's disease facts and figures. Alzheimers Dementia. 2018;14:367‐429.
-
- Prince MJ, Wimo A, Guerchet MM, Ali GC, Wu Y‐T, Prina M. World Alzheimer Report 2015 ‐ The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. Alzheimer's Disease International; 2015:82.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

