The Alzheimer's Association clinical practice guideline for the Diagnostic Evaluation, Testing, Counseling, and Disclosure of Suspected Alzheimer's Disease and Related Disorders (DETeCD-ADRD): Executive summary of recommendations for specialty care
- PMID: 39713957
- PMCID: PMC11772716
- DOI: 10.1002/alz.14337
The Alzheimer's Association clinical practice guideline for the Diagnostic Evaluation, Testing, Counseling, and Disclosure of Suspected Alzheimer's Disease and Related Disorders (DETeCD-ADRD): Executive summary of recommendations for specialty care
Abstract
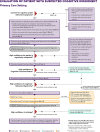
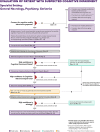
US clinical practice guidelines for the diagnostic evaluation of cognitive impairment due to Alzheimer's disease (AD) or a related dementia (ADRD) are two decades old. This evidence-based guideline was developed to empower all clinicians to implement a structured approach for evaluating a patient with symptoms that may represent clinical AD/ADRD. An expert workgroup conducted a review of 7374 publications (133 met inclusion criteria) and developed recommendations as steps in an evaluation process. This summary briefly reviews core recommendations and details specialist recommendations of a high-quality, evidence-supported evaluation process aimed at characterizing, diagnosing, and disclosing the patient's cognitive functional status, cognitive-behavioral syndrome, and likely underlying brain disease so that optimal care plans to maximize patient/care partner dyad quality of life can be developed; a companion article summarizes primary care recommendations. If clinicians use the recommendations in this guideline and health-care systems provide adequate resources, outcomes should improve in most patients in most practice settings. HIGHLIGHTS: US clinical practice guidelines for the diagnostic evaluation of cognitive impairment due to Alzheimer's disease (AD) or related dementias (ADRD) are decades old and aimed at specialists. This evidence-based guideline was developed to empower all-including primary care-clinicians to implement a structured approach for evaluating a patient with symptoms that may represent clinical AD/ADRD. This summary focuses on recommendations appropriate for specialty practice settings, forming key elements of a high-quality, evidence-supported evaluation process aimed at characterizing, diagnosing, and disclosing the patient's cognitive functional status, cognitive-behavioral syndrome, and likely underlying brain disease so that optimal care plans to maximize patient/care partner dyad quality of life can be developed; a companion article summarizes primary care recommendations. If clinicians use this guideline and health-care systems provide adequate resources, outcomes should improve in most patients in most practice settings.
Keywords: Alzheimer's disease; Lewy body dementia; cerebrospinal fluid; dementia; diagnosis; frontotemporal dementia; magnetic resonance imaging; mild cognitive impairment; molecular biomarkers; positron emission tomography; vascular cognitive impairment.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Dickerson: consulting for Acadia, Alector, Arkuda, Biogen, Eisai, Med Learning Group, Quanterix, DSMB: Lilly, Merck; royalties from Cambridge University Press, Elsevier, Oxford University Press, Up To Date. Atri: consulting for Acadia, AriBio, AZ Therapies, Biogen, Eisai, JOMDD, Lundbeck, Life Molecular Imaging, Merck, ONO, Prothena, Roche/Genentech, Novo Nordisk, Qynapse, Vaxxinity; royalties from Oxford University Press. Clevenger: none. Karlawish: DSMB for Linus Health. Knopman: DSMB for DIAN TU. Lin: consulting for Lilly. Norman: none. Onyike: consulting for Acadia Pharmaceuticals, Reata Pharmaceuticals, Otsuka Pharmaceutical, Eisai Pharmaceutical, Lykos Therapeutics, Zevra Therapeutics. Sano: consulting for Eisai, NovoNordisk, Otsuka Lundbeck. Scanland: employee of Dementia Connection, LLC; consulting for Axsome, BioXcel, Eisai, Genentech, Lundbeck, Otsuka. Carrillo: employee of Alzheimer's Association. Author disclosures are available in the supporting information.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

