Development of a Caco-2-based intestinal mucosal model to study intestinal barrier properties and bacteria-mucus interactions
- PMID: 39714032
- PMCID: PMC11702969
- DOI: 10.1080/19490976.2024.2434685
Development of a Caco-2-based intestinal mucosal model to study intestinal barrier properties and bacteria-mucus interactions
Abstract
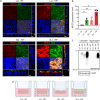
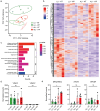
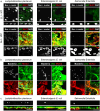
The intestinal mucosal barrier is a dynamic system that allows nutrient uptake, stimulates healthy microbe-host interactions, and prevents invasion by pathogens. The mucosa consists of epithelial cells connected by cellular junctions that regulate the passage of nutrients covered by a mucus layer that plays an important role in host-microbiome interactions. Mimicking the intestinal mucosa for in vitro assays, particularly the generation of a mucus layer, has proven to be challenging. The intestinal cell-line Caco-2 is widely used in academic and industrial laboratories due to its capacity to polarize, form an apical brush border, and reproducibly grow into confluent cell layers in different culture systems. However, under normal culture conditions, Caco-2 cultures lack a mucus layer. Here, we demonstrate for the first time that Caco-2 cultures can form a robust mucus layer when cultured under air-liquid interface (ALI) conditions on Transwell inserts with addition of vasointestinal peptide (VIP) in the basolateral compartment. We demonstrate that unique gene clusters are regulated in response to ALI and VIP single stimuli, but the ALI-VIP combination treatment resulted in a significant upregulation of multiple mucin genes and proteins, including secreted MUC2 and transmembrane mucins MUC13 and MUC17. Expression of tight junction proteins was significantly altered in the ALI-VIP condition, leading to increased permeability to small molecules. Commensal Lactiplantibacillus plantarum bacteria closely associated with the Caco-2 mucus layer and differentially colonized the surface of the ALI cultures. Pathogenic Salmonella enterica were capable of invading beyond the mucus layer and brush border. In conclusion, Caco-2 ALI-VIP cultures provide an accessible and straightforward way to culture an in vitro intestinal mucosal model with improved biomimetic features. This novel in vitro intestinal model can facilitate studies into mucus and epithelial barrier functions and in-depth molecular characterization of pathogenic and commensal microbe-mucus interactions.
Keywords: ALI; ETEC; Lactiplantibacillus plantarum; MUC2; Mucus; Salmonella enterica serovar Enteritidis; VIP; air–liquid growth; gut-on-a-chip; intestinal mucosa; tight junctions; transwell.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
