Extended Reality Gaming for Exercise and Mindfulness Throughout Pediatric Cancer Rehabilitation: Protocol for a Randomized Controlled Trial
- PMID: 39714090
- PMCID: PMC11704644
- DOI: 10.2196/64879
Extended Reality Gaming for Exercise and Mindfulness Throughout Pediatric Cancer Rehabilitation: Protocol for a Randomized Controlled Trial
Abstract
Background: Pediatric patients with cancer have limited options to self-manage their health while they are undergoing treatments in the hospital and after they are discharged to their homes. Extended reality (ER) using head-mounted displays has emerged as an immersive method of improving pain and mental health and promoting health-enhancing physical activity among a variety of clinical groups, but there is currently no established protocol for improving both physical and mental health in pediatric cancer rehabilitation.
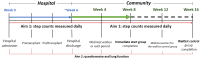
Objective: This phase I, pilot, feasibility randomized controlled trial aims to investigate the potential effects of a 14-week ER program on physical activity participation and indicators of health among pediatric patients with cancer who undergo bone marrow transplantation. An ancillary aim is to evaluate the feasibility of the program through participant engagement.
Methods: This study includes a 2-arm parallel group design with a 1-group crossover (the control group will start the intervention after a waiting period). Overall, 16 pediatric patients with cancer undergoing rehabilitation (aged ≥8 years) at a children's hospital will be randomly allocated into one of two groups: (1) an immediate start group that undergoes an ER program in the hospital until discharge and then for 8 weeks at home (total duration of approximately 14 weeks), and (2) a waitlist control group that undergoes usual care in the hospital and for 8 weeks at home, before receiving the 8-week home ER program. The program will include active video gaming with rhythmic music exercises as well as mindfulness-based practices using a high-quality app. Home-based programming will include behavioral coaching calls. Physical activity will be measured daily through step counts using a tri-axial accelerometer. Health outcomes will be measured across time and include global health, measured by the National Institutes of Health Patient-Reported Outcomes Measurement Information System (PROMIS) Pediatric Global Health Scale Short Form 7+2, and lung function, measured by a forced expiratory volume using a peak flow meter. Feasibility will be evaluated through participant engagement metrics, such as enrollment, dropout, adverse events, and attendance rates. Descriptive statistics will be obtained for all study variables. Outcomes will be modeled using mixed modeling procedures, and changes in means will be estimated with CIs.
Results: The study was funded in February 2024. Recruitment procedures started on June 27, 2024. All data are anticipated to be collected by February 2026. Full trial results are anticipated to be analyzed and submitted for publication by March 2026. The study's anticipated end date is March 31, 2026.
Conclusions: This trial tests an accessible remote program for improving both physical and mental health among pediatric patients with cancer. The knowledge obtained from this study will inform the development of a larger trial.
Trial registration: ClinicalTrials.gov NCT06298357; https://clinicaltrials.gov/study/NCT06298357.
International registered report identifier (irrid): DERR1-10.2196/64879.
Keywords: VR; XR; bone marrow transplant; exercise; extended reality; oncology; pediatric cancer; physical activity; rehabilitation; virtual reality.
©Byron Lai, Kelli Chaviano, Joshua S Richman, Mahmoud Ahmad, Ashley Wright, Raven Young, Drew Davis, James H Rimmer, Avi Madan-Swain, Joseph H Chewning. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 23.12.2024.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- Steliarova-Foucher E, Colombet M, Ries LAG, Moreno F, Dolya A, Bray F, Hesseling P, Shin HY, Stiller CA. International incidence of childhood cancer, 2001-10: a population-based registry study. Lancet Oncol. 2017;18(6):719–731. doi: 10.1016/S1470-2045(17)30186-9. http://hdl.handle.net/2318/1635135 S1470-2045(17)30186-9 - DOI - PMC - PubMed
-
- Gordon-Dseagu V, Devesa SS, Goggins M, Stolzenberg-Solomon R. Pancreatic cancer incidence trends: evidence from the surveillance, epidemiology and end results (SEER) population-based data. Int J Epidemiol. 2018;47(2):427–439. doi: 10.1093/ije/dyx232. https://europepmc.org/abstract/MED/29149259 4628151 - DOI - PMC - PubMed
-
- Butler E, Ludwig K, Pacenta HL, Klesse LJ, Watt TC, Laetsch TW. Recent progress in the treatment of cancer in children. CA Cancer J Clin. 2021;71(4):315–332. doi: 10.3322/caac.21665. https://onlinelibrary.wiley.com/doi/10.3322/caac.21665 - DOI - PubMed
-
- Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):83–103. doi: 10.3322/caac.21219. https://onlinelibrary.wiley.com/doi/10.3322/caac.21219 - DOI - PubMed
-
- Tonorezos ES, Cohn RJ, Glaser AW, Lewin J, Poon E, Wakefield CE, Oeffinger KC. Long-term care for people treated for cancer during childhood and adolescence. Lancet. 2022;399(10334):1561–1572. doi: 10.1016/S0140-6736(22)00460-3. https://europepmc.org/abstract/MED/35430023 S0140-6736(22)00460-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical