Durlobactam in combination with β-lactams to combat Mycobacterium abscessus
- PMID: 39714147
- PMCID: PMC11823594
- DOI: 10.1128/aac.01174-24
Durlobactam in combination with β-lactams to combat Mycobacterium abscessus
Abstract
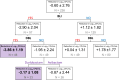
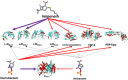
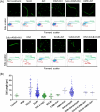
Mycobacterium abscessus (Mab) presents significant clinical challenges. This study evaluated the synergistic effects of a β-lactam and β-lactamase inhibitor combination against Mab and explored the underlying mechanisms. Synergy was assessed through MIC tests and time-kill studies, and binding affinities of nine β-lactams and BLIs to eight target receptors (L,D-transpeptidases [LDT] 1-5, D,D-carboxypeptidase, penicillin-binding protein [PBP] B, and PBP-lipo) were assessed using mass spectrometry and kinetic studies. Thermal stability and morphological changes were determined. Imipenem demonstrated high binding affinity to LDTs and PBPs, with extremely low inhibition constants (Ki,app; ≤0.002 mg/L for LDT1-2, ≤0.6 mg/L for PBPs), while cephalosporins, sulopenem, tebipenem, and amoxicillin exhibited moderate to low binding affinity. Durlobactam inactivated BlaMab and LDT/PBPs more potently than avibactam. The Ki,apps of durlobactam for PBP B, PBP-lipo, and LDT2 were below clinically achievable unbound concentrations, while avibactam's Ki,app for LDT/PBPs exceeded the clinical concentrations. Single β-lactam treatments resulted in minimal killing (~1 log10 reduction). Although avibactam yielded no effect, combinations with avibactam showed a significant reduction (~4 log10 CFU/mL). Durlobactam alone showed ~2 log10 reduction, and when combined with imipenem or two β-lactams, durlobactam achieved near-eradication of Mab, surpassing the current therapy (amikacin + clarithromycin + imipenem/cefoxitin). Inactivation of PBP-lipo by sulopenem, imipenem, durlobactam, and amoxicillin (with avibactam) led to morphological changes, showing filaments. This study demonstrates the mechanistic basis of combinations therapy, particularly imipenem + durlobactam, in overcoming β-lactam resistance in Mab.
Keywords: Mycobacterium abscessus; beta-lactamase inhibitors; beta-lactams; synergistic bacterial killing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Jhun BW, Moon SM, Jeon K, Kwon OJ, Yoo H, Carriere KC, Huh HJ, Lee NY, Shin SJ, Daley CL, Koh WJ. 2020. Prognostic factors associated with long-term mortality in 1445 patients with nontuberculous mycobacterial pulmonary disease: a 15-year follow-up study. Eur Respir J 55:55. doi:10.1183/13993003.00798-2019 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

