In vitro potentiation of tetracyclines in Pseudomonas aeruginosa by RW01, a new cyclic peptide
- PMID: 39714156
- PMCID: PMC11823630
- DOI: 10.1128/aac.01459-24
In vitro potentiation of tetracyclines in Pseudomonas aeruginosa by RW01, a new cyclic peptide
Abstract
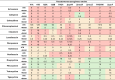
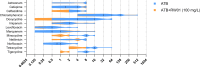
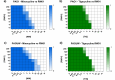
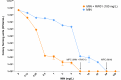
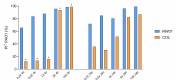
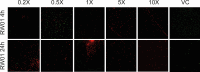
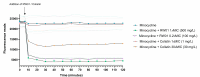
The pipeline for new drugs against multidrug-resistant Pseudomonas aeruginosa remains limited, highlighting the urgent need for innovative treatments. New strategies, such as membrane-targeting molecules acting as adjuvants, aim to enhance antibiotic effectiveness and combat resistance. RW01, a cyclic peptide with low antimicrobial activity, was selected as an adjuvant to enhance drug efficacy through membrane permeabilization. RW01's activity was evaluated via antimicrobial susceptibility testing in combination with existing antibiotics on 10 P. aeruginosa strains and analog synthesis. Synergy was assessed using checkerboard assays, and one-step mutants were generated to identify altered pathways through whole-genome sequencing and variant analysis. Permeabilizing activity was studied using flow cytometry and real-time fluorescence measurement. In vivo toxicity was assessed in female C57BL/6J mice, and possible interaction with mouse serum was also evaluated. Susceptibility testing revealed specific synergy with tetracyclines, with up to a 16-fold reduction in minimum inhibitory concentrations. Sequencing revealed that resistance to the RW01-minocycline combination involved mutations in the pmrB gene, affecting outer membrane lipopolysaccharide composition. This was further confirmed by the identification of cross-resistance to colistin in these mutants. RW01 reduced the mutant prevention concentration of minocycline from 64 to 8 mg/L. RW01 was demonstrated to enhance membrane permeabilization and therefore minocycline uptake with statistical significance. Synthetic derivatives of RW01 showed a complete loss of activity, highlighting the importance of RW01's D-proline(NH2) residue. No acute or cumulative in vivo toxicity was observed in mice. These findings suggest that RW01 could revitalize obsolete antimicrobials and potentially expand therapeutic options against multidrug-resistant P. aeruginosa.
Keywords: Pseudomonas aeruginosa; adjuvants; antimicrobial resistance; cyclic peptides; membrane disruption.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- World Health Organization . 2024. WHO bacterial priority pathogens list, 2024. Available from: https://www.who.int/publications/i/item/WHO-EMP-IAU-2024
-
- Oliver A, Rojo-Molinero E, Arca-Suarez J, Beşli Y, Bogaerts P, Cantón R, Cimen C, Croughs PD, Denis O, Giske CG, et al. 2024. Pseudomonas aeruginosa antimicrobial susceptibility profiles, resistance mechanisms and international clonal lineages: update from ESGARS-ESCMID/ISARPAE Group. Clin Microbiol Infect 30:469–480. doi: 10.1016/j.cmi.2023.12.026 - DOI - PubMed
-
- World Health Organization . 2017. Guidelines for the prevention and control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in health care facilities. Available from: http://apps.who.int/iris/bitstream/10665/259462/1/9789241550178-eng.pdf?... - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CEX2018-000806-S/Ministerio de Ciencia e Innovación (MCIN)
- 2021 SGR0 1569/Government of Catalonia | Agència de Gestió d'Ajuts Universitaris i de Recerca (AGAUR)
- IM22/INF/4/Centro de Investigación Biotecnológica en Red de Enfermedades Infecciosas (CIBERINFEC)
- PID2022-139257NB-I00/Ministerio de Ciencia e Innovación (MCIN)
- PI21/00017/MEC | Instituto de Salud Carlos III (ISCIII)
LinkOut - more resources
Full Text Sources
Medical

