Single-chain antibody gene therapy strategy based on high-throughput screening triggers sustained antiviral activity in the body
- PMID: 39714166
- PMCID: PMC11784017
- DOI: 10.1128/jvi.01497-24
Single-chain antibody gene therapy strategy based on high-throughput screening triggers sustained antiviral activity in the body
Abstract
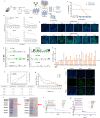
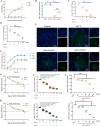
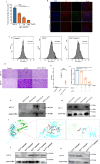
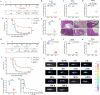
The occurrence of viral diseases poses a huge threat and impact on human public health safety and the development of the animal and fishery industry. Here, a strain of single-chain antibody fragment, scFv-1, was isolated from the phage antibody display library construct by immunizing New Zealand white rabbits with rhabdovirus. In vitro analysis showed that the single-chain antibody could inhibit the infection of the virus in multiple pathways, including adsorption, fusion, and release. In vivo analysis revealed scFv-1 had a preventive and protective effect against the infection of virus. In addition, we describe that transposon-based transport of neutralizing genes allows for long-term, continuous expression, avoiding the need for lifelong, repeated passive immunization for treatment. In sum, high-throughput screening of neutralization genes based on phage display technology and transposon vector-based gene transfer provides effective methods for treating and preventing diseases and avoiding repetitive passive immunotherapy. This study also provides a reference for the prevention and treatment of unknown pathogens.IMPORTANCELivestock and fisheries play an important role in economic development and food security. The frequent outbreaks of viral diseases have caused great losses to the livestock industry, while the increase in drug resistance caused by the use of antibiotics as well as the potential risks to human health have raised serious concerns. Here, we constructed a phage display antibody library by immunizing New Zealand white rabbits with purified rhabdovirus and selected a single-chain antibody, scFv-1, with good neutralizing activity, which was validated and found to be able to block multiple phases of the virus and thus play a neutralizing role. In addition, we describe that transposon-based transport of neutralizing genes allows for long-term, continuous expression, reducing the need for lifelong, repeated passive immunization for treatment. Our work not only provides methods for the prevention and treatment of viral diseases but also provides the body with long-lasting and even permanent protection against repeated passive immunization.
Keywords: antibody gene transfer; neutralization gene therapy; single-chain antibodies; viral diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
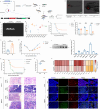
References
-
- Newman J, Thakur N, Peacock TP, Bialy D, Elrefaey AME, Bogaardt C, Horton DL, Ho S, Kankeyan T, Carr C, Hoschler K, Barclay WS, Amirthalingam G, Brown KE, Charleston B, Bailey D. 2022. Neutralizing antibody activity against 21 SARS-CoV-2 variants in older adults vaccinated with BNT162b2. Nat Microbiol 7:1180–1188. doi: 10.1038/s41564-022-01163-3 - DOI - PMC - PubMed
-
- Guo Y, Zhang G, Yang Q, Xie X, Lu Y, Cheng X, Wang H, Liang J, Tang J, Gao Y, et al. 2023. Discovery and characterization of potent pan-variant SARS-CoV-2 neutralizing antibodies from individuals with Omicron breakthrough infection. Nat Commun 14:3537. doi: 10.1038/s41467-023-39267-x - DOI - PMC - PubMed
-
- Suryadevara N, Shrihari S, Gilchuk P, VanBlargan LA, Binshtein E, Zost SJ, Nargi RS, Sutton RE, Winkler ES, Chen EC, Fouch ME, Davidson E, Doranz BJ, Chen RE, Shi P-Y, Carnahan RH, Thackray LB, Diamond MS, Crowe JE Jr. 2021. Neutralizing and protective human monoclonal antibodies recognizing the N-terminal domain of the SARS-CoV-2 spike protein. Cell 184:2316–2331. doi: 10.1016/j.cell.2021.03.029 - DOI - PMC - PubMed
-
- Pegu A, Borate B, Huang Y, Pauthner MG, Hessell AJ, Julg B, Doria-Rose NA, Schmidt SD, Carpp LN, Cully MD, Chen X, Shaw GM, Barouch DH, Haigwood NL, Corey L, Burton DR, Roederer M, Gilbert PB, Mascola JR, Huang Y. 2019. A meta-analysis of passive immunization studies shows that serum-neutralizing antibody titer associates with protection against SHIV challenge. Cell Host Microbe 26:336–346. doi: 10.1016/j.chom.2019.08.014 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

