Prognostic serum biomarkers of synaptic, neuronal and glial injury in patients with acute ischemic stroke of the anterior circulation
- PMID: 39714176
- PMCID: PMC11664725
- DOI: 10.1111/ene.16581
Prognostic serum biomarkers of synaptic, neuronal and glial injury in patients with acute ischemic stroke of the anterior circulation
Abstract
Background: We aimed to investigate the prognostic role of β-synuclein in comparison to that of neurofilament light chain (NfL) and glial fibrillary acidic protein (GFAP) for predicting functional outcome after acute ischemic stroke (AIS).
Methods: We measured serum concentrations of β-synuclein, NfL and GFAP 24 h after hospital admission in 213 consecutive patients with moderate-to-severe AIS. We investigated the association between serum biomarkers and radiological/clinical characteristics, 3-months mortality and functional outcome on the modified Rankin Scale (mRS).
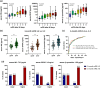
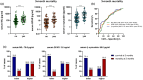
Results: In 213 patients with AIS [mean age: 76.1 (±12.5) years, 53.1% males, median NIHSS score on admission: 13 (IQR: 9-17)], higher levels of β-synuclein, NfL and GFAP were associated with higher NIHSS scores and with lower Alberta Stroke Program CT Score (ASPECTS) points on admission. Serum β-synuclein levels was significantly correlated with NfL (rho = 0.715, p < 0.001) and GFAP concentrations (rho = 0.684, p < 0.001). The inclusion of serum β-synuclein significantly improved the accuracy of prediction models without biomarkers for overall mortality (AUC: 0.836 vs. 0.752, p < 0.001) and mRS 3-6 vs. 0-2 (AUC: 0.812 vs. 0.624, p < 0.001). Combination models with NfL and/or GFAP showed a similar accuracy.
Conclusions: Serum β-synuclein may be used to assess synaptic damage/dysfunction and to predict 3-months clinical outcomes in patients with AIS.
Keywords: GFAP; NfL; beta‐synuclein; biomarkers; stroke.
© 2024 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
MO gave scientific advice for Fujirebio, Roche, Biogen, and Axon; MO, SH and PO are co‐inventors of a patent handed for the measurement of beta‐synuclein. KGH reports speaker's honoraria, consulting fees, lecture honoraria and/or study grants from Abbott, Alexion, Amarin, AstraZeneca, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol‐Myers Squibb, Daiichi Sankyo, Edwards Lifesciences, Medronic, Novartis, Pfizer, Portola, Premier Research, Sanofi, SUN Pharma, and W.L. Gore and Associates. PUH reports grants from German Ministry of Research and Education, German Research Foundation, European Union, Federal Joint Committee (G‐BA) within the Innovationfond, Berlin Chamber of Physicians, German Parkinson Society, University Hospital Würzburg, Robert Koch Institute, German Heart Foundation, University Göttingen (within FIND‐AF [A Prospective, Randomised, Controlled Study to Determine the Detection of Atrial Fibrillation by Prolonged and Enhanced Holter Monitoring as Compared to Usual Care in Stroke Patients], supported by an unrestricted research grant to the University Göttingen from Boehringer‐Ingelheim), University Hospital Heidelberg (within Registry of Acute Stroke Under Novel Oral Anticoagulants [RASUNOA]‐prime, supported by an unrestricted research grant to the University Hospital Heidelberg from Bayer, Bristol‐Myers Squibb, Boehringer‐Ingelheim, Daiichi Sankyo), Charité–Universitätsmedizin Berlin (within MonDAFIS, supported by an unrestricted research grant to the Charité from Bayer), all outside the submitted work. PO received research support from the Cure Alzheimer Fund, ALS Association (24‐SGP‐691, 23‐PPG‐674‐2), ALS Finding a Cure, the Charcot Foundation, the DZNE Innovation‐to‐Application program and consulting fees from LifeArc and Fundamental Pharma. The other authors have nothing to declare.
Figures

References
-
- Vollmuth C, Fiessler C, Montellano FA, et al. Incremental value of serum neurofilament light chain and glial fibrillary acidic protein as blood‐based biomarkers for predicting functional outcome in severe acute ischemic stroke. Eur Stroke J. 2024;9:751‐762. doi:10.1177/23969873241234436 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

