Identification of an antibiotic from an HTS targeting EF-Tu:tRNA interaction: a prospective topical treatment for MRSA skin infections
- PMID: 39714192
- PMCID: PMC11784183
- DOI: 10.1128/aem.02046-24
Identification of an antibiotic from an HTS targeting EF-Tu:tRNA interaction: a prospective topical treatment for MRSA skin infections
Abstract
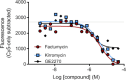
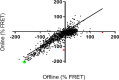
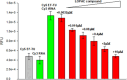
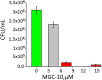
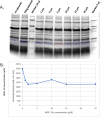
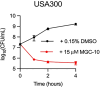
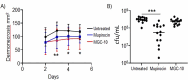
Because of the urgent need for new antibiotics to treat drug-resistant bacterial pathogens, we employed an assay that rapidly screens large quantities of compounds for their ability to interfere with bacterial protein synthesis, in particular, the delivery of amino acids to the ribosome via tRNA and elongation factor Tu (EF-Tu). We have identified a drug lead, named MGC-10, which kills Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA), with a MIC of 6 µM, while being harmless to mammalian cells in vitro in that concentration range. The antibacterial activity of MGC-10 was broad against over 50 strains of antibiotic-resistant samples obtained from hospital infections, where MGC-10 inhibited all tested strains of MRSA. Extensive selection and screening with MGC-10 did not yield any resistant strains, indicating it may have universal antibacterial activity against S. aureus. Pharmacokinetics performed in mice suggested that MGC-10 was too toxic for systemic use; however, it appears to have potential as a topical treatment for difficult-to-treat wounds or skin infections by Gram-positive pathogens such as MRSA. In a mouse skin-infection model with MRSA, MGC-10 performed as well or better than the present topical drug of choice, mupirocin. MGC-10 showed little, if any, accumulation in the livers of topically treated mice. These results bode well for the future use of MGC-10 in clinical application as it could be used to treat a broad range of S. aureus skin infections that are resistant to known antibiotics.IMPORTANCEThere is a critical need for new antibiotics to treat bacterial infections caused by pathogens resistant to many if not all currently available antibiotics. We describe here the identification of a prospective new antibiotic from high-throughput screening of a chemical library. The screening was designed to detect the inhibition of formation of a complex required for bacterial protein synthesis in all bacteria, the "ternary complex," comprised of elongation factor Tu (EF-Tu), aminoacyl-tRNA, and GTP. The inhibitory compound, renamed MGC-10, was effective against all Gram-positive bacteria, including a wide variety of methicillin-resistant Staphylococcus aureus (MRSA) strains. Although apparently too toxic for systemic use, the compound was safe and effective for topical use for treating skin infections in a mouse model. No resistance to the compound has been detected thus far, suggesting the potential to develop this compound for topical use to treat infections, especially those caused by pathogens resistant to existing antibiotics.
Keywords: (R,R)-tetrahydrochrysene; FRET; Staphylococcus aureus; ternary complex in protein synthesis; topical antibiotic for Gram-positive pathogens.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

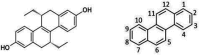

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

