Exploiting gasdermin-mediated pyroptosis for enhanced antimicrobial activity of phage endolysin against Pseudomonas aeruginosa
- PMID: 39714210
- PMCID: PMC11748493
- DOI: 10.1128/msystems.01106-24
Exploiting gasdermin-mediated pyroptosis for enhanced antimicrobial activity of phage endolysin against Pseudomonas aeruginosa
Abstract
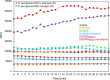
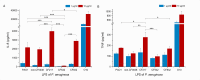
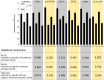
Pyroptosis is an inflammatory immune response of eukaryotic cells to bacterial lipopolysaccharide (LPS) and other pathological stimuli, leading to the activation of the gasdermin D (GSDMD) and secretion of pore-forming domain GSDMDNterm, facilitating the release of cytokines. Additionally, GSDMDNterm exhibits antibacterial properties through interactions with bacterial outer membranes (OM). We explored alternative antimicrobial strategy to determine whether inducing natural pyroptosis via GSDMD activation by P. aeruginosa LPS could enhance the effectiveness of recombinant phage endopeptidase KP27 (peptidoglycan-degrading enzyme) against P. aeruginosa, enabling penetration through OM and bacterial killing synergistically. Our findings demonstrated that recombinant GSDMD alone exhibited antibacterial effects against wild-type P. aeruginosa with smooth LPS, while recombinant GSDMDNterm efficiently permeabilized both smooth LPS-bearing and O-chain-deficient P. aeruginosa potentially synergizing with endolysin KP27. Transcriptomic analyses revealed the activation of the immune system pathways in response to LPS, mainly in monocytic cells, in contrast to epithelial A549 or HeLa cell lines. LPS-induced pyroptosis in monocytes led to GSDMD cleavage and the release of interleukins, regardless of the nature/origin of the LPS used. However, the pyroptosis stimulation by LPS in the antibacterial assay was not effective enough for bacterial OM permeabilization and enhancement of endolysin activity. We assume that leveraging pyroptosis induction in monocytic cells to augment the bactericidal activity of endolysins may be limited.
Importance: Recombinant GSDMDNterm protein was able to efficiently permeabilize P. aeruginosa outer membranes and increase endolysin activity against bacteria, producing either long LPS O-chains or lack them entirely. The obtained results suggest the limited possibility of using the natural process of pyroptosis occurring in monocytic cells to enhance the bactericidal effect of recombinant phage endolysins against Gram-negative bacteria infection.
Keywords: Pseudomonas aeruginosa LPS; endolysin; gasdermin D; outer membrane permeabilization; pyroptosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
The influence of cationic dendrimers on antibacterial activity of phage endolysin against P. aeruginosa cells.Bioorg Chem. 2019 Oct;91:103121. doi: 10.1016/j.bioorg.2019.103121. Epub 2019 Jul 11. Bioorg Chem. 2019. PMID: 31310881
-
Pyroptosis inhibition alleviates acute lung injury via E-twenty-six variant gene 5-mediated downregulation of gasdermin D.Respir Physiol Neurobiol. 2025 Jan;331:104346. doi: 10.1016/j.resp.2024.104346. Epub 2024 Sep 11. Respir Physiol Neurobiol. 2025. PMID: 39265817
-
Wedelolactone ameliorates Pseudomonas aeruginosa-induced inflammation and corneal injury by suppressing caspase-4/5/11/GSDMD-mediated non-canonical pyroptosis.Exp Eye Res. 2021 Oct;211:108750. doi: 10.1016/j.exer.2021.108750. Epub 2021 Sep 2. Exp Eye Res. 2021. PMID: 34481822
-
New insights into Gasdermin D pore formation.Biochem Soc Trans. 2024 Apr 24;52(2):681-692. doi: 10.1042/BST20230549. Biochem Soc Trans. 2024. PMID: 38497302 Review.
-
The Role of Gasdermin-D-Mediated Pyroptosis in Organ Injury and Its Therapeutic Implications.Organogenesis. 2023 Dec 31;19(1):2177484. doi: 10.1080/15476278.2023.2177484. Organogenesis. 2023. PMID: 36967609 Free PMC article. Review.
References
-
- Qin S, Xiao W, Zhou C, Pu Q, Deng X, Lan L, Liang H, Song X, Wu M. 2022. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Sig Transduct Target Ther 7:1–27. doi:10.1038/s41392-022-01056-1 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

