Diversification of single-cell growth dynamics under starvation influences subsequent reproduction in a clonal bacterial population
- PMID: 39714219
- PMCID: PMC11773413
- DOI: 10.1093/ismejo/wrae257
Diversification of single-cell growth dynamics under starvation influences subsequent reproduction in a clonal bacterial population
Abstract
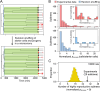
Most of the microbes in nature infrequently receive nutrients and are thus in slow- or non-growing states. How quickly they can resume their growth upon an influx of new resources is crucial to occupy environmental niches. Isogenic microbial populations are known to harbor only a fraction of cells with rapid growth resumption, yet little is known about the physiological characteristics of those cells and their emergence in the population. Here, we tracked growth of individual Escherichia coli cells in populations under fluctuating nutrient conditions. We found that shifting from high- to low-nutrient conditions caused stalling of cell growth with few cells continuing to divide extremely slowly, a process which was dependent on lipid turnover. Resuming high-nutrient inflow after low-nutrient conditions resulted in cells resuming growth and division, but with different lag times and leading to varying progeny. The history of cell growth during low-nutrient but not high-nutrient conditions was determinant for resumption of growth, which cellular genealogy analysis suggested to originate from inherited physiological differences. Our results demonstrate that cellular growth dynamics become diverse by nutrient limitations, under which a fraction of cells experienced a particular growth history can reproduce progeny with new resources in the future.
Keywords: growth dynamics; single cell; starvation; time-lapse imaging.
© The Author(s) 2024. Published by Oxford University Press on behalf of the International Society for Microbial Ecology.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Stearns SC. Trade-offs in life-history evolution. Funct Ecol 1989;3:259–68. 10.2307/2389364 - DOI
-
- Levins R. Evolution in Changing Environments; some Theoretical Explorations. Prinston: Princeton University Press, 1968, 10.1515/9780691209418. - DOI
-
- Colwell RR, Brayton PR, Grimes DJet al. Viable but non-culturable vibrio cholerae and related pathogens in the environment: implications for release of genetically engineered microorganisms. Nat Biotechnol 1985;3:817–20. 10.1038/nbt0985-817 - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

