Phytochemistry, Antibacterial and Antioxidant Activities of Grewia lasiocarpa E. Mey. Ex Harv. Fungal Endophytes: A Computational and Experimental Validation Study
- PMID: 39714366
- PMCID: PMC12081030
- DOI: 10.1002/cbdv.202402908
Phytochemistry, Antibacterial and Antioxidant Activities of Grewia lasiocarpa E. Mey. Ex Harv. Fungal Endophytes: A Computational and Experimental Validation Study
Abstract
The genus Grewia are well-known for their medicinal properties and are widely used in traditional remedies due to their rich phytochemical composition and potential health benefits. This study isolated and characterized five endophytic fungi from Grewia lasiocarpa E. Mey. Ex Harv. and evaluated their in vitro antibacterial and antioxidant activities. Five [Aspergillus fumigatus (MK243397.1), A. fumigatus (MK243451.1), Penicillium raistrickii (MK243492.1), P. spinulosum (MK243479.1), Meyerozyma guilliermondii (MK243634.1)] of the 22 isolated endophytic fungi had inhibitory activity (62.5-1000 µg/mL) against methicillin-resistant Staphylococcus aureus (MRSA). The antioxidant activities were 66.5% and 98.4% for 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ferric ion reducing antioxidant power (FRAP), respectively. In silico evaluation of the phytochemicals of the extract (containing majorly n-hexadecanoic acid) was performed against penicillin-binding protein 2a (PBP2a) implicated in the broad clinical resistance of MRSA to conventional beta-lactams. Molecular docking and molecular dynamic simulation analyses revealed that the phytosterol constituents of the extract, especially dehydroergosterol (-46.28 kcal/mol), had good stability (4.35 Å) and compactness (35.08 Å) with PBP2a relative to the unbound PBP2a and amoxicillin-PBP2a complex during the 100 ns simulation period, reinforcing them as putative leads that may be developed as viable alternatives to beta-lactams against infections caused by MRSA. However, the prediction that dehydroergosterol lacks oral bioavailability with poor water solubility suggests that it could benefit from structural optimization for improved druggability. Hence, isolating and derivatizing dehydroergosterol for subsequent evaluation against PBP2a in vitro and in vivo is highly recommended.
Keywords: Aspergillus sp; Meyerozyma guilliermondii; Penicillium sp; methicillin‐resistant Staphylococcus aureus; molecular docking; penicillin‐binding protein 2a (PBP2a).
© 2025 The Author(s). Chemistry & Biodiversity published by Wiley‐VHCA AG.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


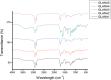





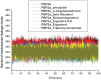
References
-
- a) Iwu M. W., Duncan A. R., and Okunji C. O., “New Antimicrobials of Plant Origin,” in Perspectives on New Crops and New Uses, ed. Janick J. (Alexandria, VA: ASHS Press, 1999), 457–462.
- b) Baral B., Mamale K., Gairola S., Chauhan C., Dey A., and Kaundal R. K., “Infectious Diseases and Its Global Epidemiology,” in Nanostructured Drug Delivery Systems in Infectious Disease Treatment, eds. Beg S., Shukla R., Handa M., Rahman M., and Dhir A. (Amsterdam: Academic Press, 2024), 1–24, 10.1016/B978-0-443-13337-4.00017-3. - DOI
-
- a) Wise R., “The Worldwide Threat of Antimicrobial Resistance,” Current Science 95 (2008): 181–187. Accessed December 28, 2024, http://www.jstor.org/stable/2410304.
- b) Abdallah E. M., Alhatlani B. Y., de Paula Menezes R., and Martins C. H. G., “Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post‐Antibiotic Era,” Plants 12 (2023): 3077. - PMC - PubMed
- c) Ožegić O., Bedenić B., Sternak S. L., et al., “Antimicrobial Resistance and Sports: The Scope of the Problem, Implications for Athletes' Health and Avenues for Collaborative Public Health Action,” Antibiotics 13 (2024): 232. - PMC - PubMed
-
- a) Mathur V. and Ulanova D., “Microbial Metabolites Beneficial to Plant Hosts Across Ecosystems,” Microbial Ecology 86 (2023): 25–48. - PubMed
- b) Selim M. S. M., Abdelhamid S. A., and Mohamed S. S., “Secondary Metabolites and Biodiversity of Actinomycetes,” Journal of Genetic Engineering and Biotechnology 19 (2021): 72. - PMC - PubMed
-
- Mishra S., Bhattacharjee A., and Sharma S., “An Ecological Insight into the Multifaceted World of Plant–Endophyte Association,” Critical Reviews in Plant Sciences 40 (2021): 127–146.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

