Role of arbutin in the inhibition of FBXO5 in hepatocellular carcinoma
- PMID: 39714552
- PMCID: PMC11666847
- DOI: 10.1007/s12672-024-01729-z
Role of arbutin in the inhibition of FBXO5 in hepatocellular carcinoma
Abstract
Purpose: This work investigated the effect of FBXO5 in hepatocellular carcinoma (HCC) and the mechanism of action of arbutin in its inhibition.
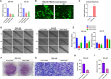
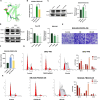
Methods: FBXO5 mRNA and protein expressions in the tumor were assessed using TCGA, ICGC and HPA databases. Cox regression analysis and Kaplan-Meier survival curves were employed to assess the impact of FBXO5 on the survival outcomes of patients with HCC. Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), Gene Set Enrichment Analysis (GSEA), and Gene Set Variation Analysis (GSVA) were used to investigate the biological function associated with FBXO5-related genes. The role of FBXO5 as oncogene and the inhibitory mechanism of arbutin were confirmed through western blotting (WB), reverse transcription quantitative polymerase chain reaction (RT-qPCR), and in vitro experiments such as scratch wound-healing migration assay, plate clone formation assay, and transwell migration assay.
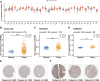
Results: Patients with high FBXO5 expression showed shorted overall survival (OS), progression-free survival (PFS), disease-specific survival (DSS), and disease-free survival (DFS). FBXO5 was identified as an independent prognostic risk factor associated with the cell cycle. In vitro investigations indicated that FBXO5 facilitated HCC progression by modulating the cell cycle, while arbutin suppressed FBXO5 expression and regulated cell cycle dynamics.
Conclusion: FBXO5 is a potential diagnostic and prognostic biomarker for HCC, and arbutin may exert anticancer effects through the suppression of FBXO5 expression.
Keywords: Arbutin; Cell cycle; FBXO5; HCC.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare that they do not have any competing interests.
Figures



Similar articles
-
UBR1 is a prognostic biomarker and therapeutic target associated with immune cell infiltration in gastric cancer.Aging (Albany NY). 2024 Aug 23;16(16):12029-12049. doi: 10.18632/aging.206079. Epub 2024 Aug 23. Aging (Albany NY). 2024. PMID: 39181686 Free PMC article.
-
Identification of Rad51 as a prognostic biomarker correlated with immune infiltration in hepatocellular carcinoma.Bioengineered. 2021 Dec;12(1):2664-2675. doi: 10.1080/21655979.2021.1938470. Bioengineered. 2021. PMID: 34115569 Free PMC article.
-
Overexpressed GNAZ predicts poor outcome and promotes G0/G1 cell cycle progression in hepatocellular carcinoma.Gene. 2022 Jan 10;807:145964. doi: 10.1016/j.gene.2021.145964. Epub 2021 Sep 14. Gene. 2022. PMID: 34530087
-
Over-expression of RRM2 predicts adverse prognosis correlated with immune infiltrates: A potential biomarker for hepatocellular carcinoma.Front Oncol. 2023 Mar 28;13:1144269. doi: 10.3389/fonc.2023.1144269. eCollection 2023. Front Oncol. 2023. PMID: 37056349 Free PMC article.
-
ELOVLs Predict Distinct Prognosis Value and Immunotherapy Efficacy In Patients With Hepatocellular Carcinoma.Front Oncol. 2022 Jul 15;12:884066. doi: 10.3389/fonc.2022.884066. eCollection 2022. Front Oncol. 2022. PMID: 35912257 Free PMC article.
References
-
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–14. 10.1016/S0140-6736(18)30010-2. - PubMed
-
- Gao L, Wu ZX, Assaraf YG, Chen ZS, Wang L. Overcoming anti-cancer drug resistance via restoration of tumor suppressor gene function. Drug Resist Updat. 2021;57: 100770. 10.1016/j.drup.2021.100770. - PubMed
-
- Soerjomataram I, Oomen D, Lemmens V, Oenema A, Benetou V, Trichopoulou A, Coebergh JW, Barendregt J, de Vries E. Increased consumption of fruit and vegetables and future cancer incidence in selected European countries. Eur J Cancer. 2010;46:2563–80. 10.1016/j.ejca.2010.07.026. - PubMed
-
- Garg P, Garg R, Horne D, Awasthi S, Salgia R, Singhal SS. Prognostic significance of natural products against multidrug tumor resistance. Cancer Lett. 2023;557: 216079. 10.1016/j.canlet.2023.216079. - PubMed
Grants and funding
- S202310367124/the Anhui Provincial College Students' Innovation Training Programme Project
- S202310367124/the Anhui Provincial College Students' Innovation Training Programme Project
- S202310367124/the Anhui Provincial College Students' Innovation Training Programme Project
- S202310367124/the Anhui Provincial College Students' Innovation Training Programme Project
- KJ2021A0711/the Scientific Research Fund of Anhui Provincial Department of Education
LinkOut - more resources
Full Text Sources
