A prospective study comparing highly qualified Molecular Tumor Boards with AI-powered software as a medical device
- PMID: 39714567
- PMCID: PMC11785689
- DOI: 10.1007/s10147-024-02684-z
A prospective study comparing highly qualified Molecular Tumor Boards with AI-powered software as a medical device
Abstract
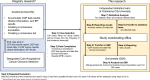
Background: The implementation of cancer precision medicine in Japan is deeply intertwined with insurance reimbursement policies and requires case-by-case reviews by Molecular Tumor Boards (MTBs), which impose considerable operational burdens on healthcare facilities. The extensive preparation and review times required by MTBs hinder their ability to efficiently assess comprehensive genomic profiling (CGP) test results. Despite attempts to optimize MTB operations, significant challenges remain. This study aims to evaluate the effectiveness of QA Commons, an artificial intelligence-driven system designed to improve treatment planning using CGP analysis. QA Commons utilizes a comprehensive knowledge base of drugs, regulatory approvals, and clinical trials linked to genetic biomarkers, thereby enabling the delivery of consistent and standardized treatment recommendations. Initial assessments revealed that the QA Commons' recommendations closely matched the ideal treatment recommendations (consensus annotations), outperforming the average results of MTBs at Cancer Genomic Medicine Core Hospitals.
Methods: A clinical performance evaluation study will be conducted by comparing the QA Commons' treatment recommendations with those of the Academia Assembly, which includes medical professionals from the Cancer Genomic Medicine Core and Hub Hospitals. One hundred cases selected from the "Registry of the Academia Assembly," based on defined inclusion and exclusion criteria, will be analyzed to assess the concordance of recommendations.
Conclusion: The expected outcomes suggest that QA Commons could reduce the workload of MTB members, standardize the quality of MTB discussions, and provide consistent outcomes in repeated patient consultations. In addition, the global expansion of QA Commons could promote worldwide adoption of Japan's pioneering precision oncology system.
Keywords: AI-powered diagnostic tools; Cancer precision medicine; Comprehensive genomic profiling; Molecular Tumor Board.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interests: Hideaki Bando received research funding from Ono Pharmaceutical and honoraria from Ono Pharmaceutical, Taiho Pharmaceutical, and Eli Lilly Japan. Yoichi Naito received research funding from ABBVIE, Ono, Daiichi Sankyo, Taiho, Pfizer, Boehringer Ingelheim, Eli Lilly, Eisai, AstraZeneca, Chugai, and Baye, and honoraria from AstraZeneca, Eisai, Ono, Guardant, Takeda, Eli Lilly, Novartis, Pfizer, Chugai, PDR Pharma, Nihon Kayaku, Taiho, Bristol Mayers, Bayer, and Dai@@. Tomoyuki Yamada is an employee of Genomedia Inc. Takao Fujisawa received honoraria for lectures from Amelieff Co., Ltd. Mitsuho Imai is an advisory for Sumitomo Co., Ltd. and Exact Sciences Corporation, speakers’ bureau from Chugai Pharmaceutical Co., Ltd., and received research funding from Merck Sharp and Dohme, Inc. Yasutoshi Sakamoto and Yusuke Saigusa declare no conflicts of interest. Koji Yamamoto received research funding from Chugai Pharmaceutical and honoraria from FUJIFILM, Toyama, Delta-Fly Pharma, Takeda, CMIC, CM Plus, DAIICHI SANKYO, AstraZeneca, and TME. Yutaka Tomioka and Nobuyoshi Takeshita report no conflict of interest. Kuniko Sunami received research funding from Sysmex. Megumi Futamura and Chiemi Notake report no conflicts of interest. Satoko Aoki and Kazunori Okano are employees of Genomedia, Inc. Takayuki Yoshino received honoraria from Taiho Pharmaceutical, Chugai Pharmaceutical, Eli Lilly, Merck Biopharma, Bayer Yakuhin, Ono Pharmaceutical, and MSD and research funding from Taiho Pharmaceutical, Ono Pharmaceutical, Chugai Pharmaceutical, Amgen, Parexel International, MSD, Daiichi Sankyo, Pfizer, Genomedia, Sysmex, Nihon Boehringer Ingelheim, and Sanofi. Ethical approval: The trial protocol was approved by the institutional review board of each participating site before study initiation (2024-230). The study will be conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan.
Figures


References
-
- Kohno T, Kato M, Kohsaka S et al (2022) C-CAT: the national datacenter for cancer genomic medicine in japan. Cancer Discov 12:2509–2515. 10.1158/2159-8290.CD-22-0417 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

