Development and evaluation of deuterated [18F]JHU94620 isotopologues for the non-invasive assessment of the cannabinoid type 2 receptor in brain
- PMID: 39714717
- PMCID: PMC11666850
- DOI: 10.1186/s41181-024-00319-2
Development and evaluation of deuterated [18F]JHU94620 isotopologues for the non-invasive assessment of the cannabinoid type 2 receptor in brain
Abstract
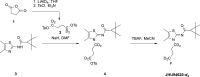
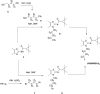
Background: The cannabinoid type 2 receptors (CB2R) represent a target of increasing importance in neuroimaging due to its upregulation under various neuropathological conditions. Previous evaluation of [18F]JHU94620 for the non-invasive assessment of the CB2R availability by positron emission tomography (PET) revealed favourable binding properties and brain uptake, however rapid metabolism, and generation of brain-penetrating radiometabolites have been its main limitations. To reduce the bias of CB2R quantification by blood-brain barrier (BBB)-penetrating radiometabolites, we aimed to improve the metabolic stability by developing -d4 and -d8 deuterated isotopologues of [18F]JHU94620.
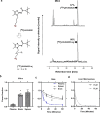
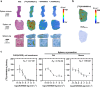
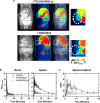
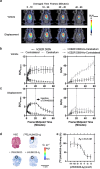
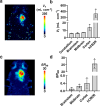
Results: The deuterated [18F]JHU94620 isotopologues showed improved metabolic stability avoiding the accumulation of BBB-penetrating radiometabolites in the brain over time. CB2R-specific binding with KD values in the low nanomolar range was determined across species. Dynamic PET studies revealed a CB2R-specific and reversible uptake of [18F]JHU94620-d8 in the spleen and to a local hCB2R(D80N) protein overexpression in the striatal region in rats.
Conclusion: These results support further investigations of [18F]JHU94620-d8 in pathological models and tissues with a CB2R overexpression as a prerequisite for clinical translation.
Keywords: Cannabinoid type 2 receptor; Neuroinflammation; Positron emission tomography; [18F]JHU94620.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All studies involving animals were carried out according to the national law on the protection of animals and were approved by the responsible authorities (Landesdirektion Sachsen, No. DD24.1-5131/446/19, TVV 18/18), CD-1 mice and Wistar rats were obtained from the Medizinisch-Experimentelles-Zentrum at Universität Leipzig (Leipzig, Germany) and a pig (German Landrace x German Large White) was obtained from the Lehr- und Versuchsgut Oberholz (Großpösna, Germany). Animals were kept under standard conditions with free access to water and food. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures
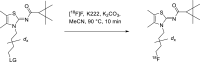
References
-
- Auvity S, Attili B, Caillé F, Goislard M, Cayla J, Hinnen F, et al. Translational Preclinical PET Imaging and Metabolic Evaluation of a New Cannabinoid 2 Receptor (CB2R) Radioligand, (Z)-N-(3-(2-(2-[18F]Fluoroethoxy)ethyl)-4,5-dimethylthiazol-2(3H)-ylidene)-2,2,3,3-tetramethylcyclopropane-1-carboxamide. ACS Pharmacol Transl Sci. 2024;7:3144–54. 10.1021/acsptsci.4c00348. - DOI - PMC - PubMed
-
- Benito C, Núñez E, Tolón RM, Carrier EJ, Rábano A, Hillard CJ, et al. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J Neurosci. 2003;23:11136–41. 10.1523/JNEUROSCI.23-35-11136.2003. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
