Clinical Outcomes of Early Phenotype-Desirable Antimicrobial Therapy for Enterobacterales Bacteremia
- PMID: 39714841
- PMCID: PMC11667351
- DOI: 10.1001/jamanetworkopen.2024.51633
Clinical Outcomes of Early Phenotype-Desirable Antimicrobial Therapy for Enterobacterales Bacteremia
Abstract
Importance: Initiating effective therapy early is associated with improved survival among patients hospitalized with gram-negative bloodstream infections; furthermore, providing early phenotype-desirable antimicrobial therapy (PDAT; defined as receipt of a β-lactam antibiotic with the narrowest spectrum of activity to effectively treat the pathogen's phenotype) is crucial for antimicrobial stewardship. However, the timing of targeted therapy among patients hospitalized with gram-negative bloodstream infections is not well understood.
Objective: To compare the clinical outcomes between patients who were hospitalized with Enterobacterales bloodstream infections receiving early vs delayed PDAT.
Design, setting, and participants: This retrospective cohort study used a large, geographically diverse, hospital-based US database (PINC AI Healthcare Database). Participants were adult (aged ≥18 years) patients with an inpatient admission between January 1, 2017, and June 30, 2022, with at least 1 blood culture isolate positive for Escherichia coli, Klebsiella oxytoca, Klebsiella pneumoniae, or Proteus mirabilis and receiving PDAT on blood culture collection days 0 to 4.
Exposure: Early vs delayed PDAT, with early PDAT defined as receipt of PDAT on blood culture collection days 0 to 2.
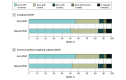
Main outcomes and measures: The main outcome was desirability of outcome ranking, in which patients were assigned a mutually exclusive rank 1 through 5. Rank 1 indicated the most desirable outcome (alive with no events), whereas rank 5 indicated the least desirable outcome and included all patients who died within 30 days of blood culture collection.
Results: Among 8193 eligible patients (mean [SD] age, 69.0 [16.4] years; 4758 [58.1%] female; 1200 [14.6%] African American or Black, 729 [8.9%] Hispanic, and 5778 [70.5%] White) from 252 hospitals, 5033 (61.4%) received early PDAT. Patients receiving early PDAT were similar in age (mean [SD], 68.2 [16.9] vs 70.3 [15.6] years) but more likely to have a lower median (IQR) Charlson-Deyo comorbidity score (2 [1-5] vs 3 [1-5]) compared with patients receiving delayed PDAT. After adjusting for comorbidities and severity of illness, patients receiving early PDAT were 20% less likely to be readmitted within 30 days compared with those receiving delayed PDAT (odds ratio, 0.80; 95% CI, 0.69-0.92; P < .001). A higher percentage of patients receiving early PDAT had a desirability of outcome ranking of 1 compared with patients receiving delayed PDAT (56.3% vs 52.2%, P < .001). Those receiving early PDAT had a 52.5% probability (95% CI, 51.3%-53.7%) of a more desirable outcome than those receiving delayed PDAT, a finding that persisted in the adjusted analysis (probability, 52.0%; 95% CI, 50.9%-53.2%).
Conclusions and relevance: Receiving early PDAT was associated with favorable 30-day clinical outcomes among patients hospitalized with Enterobacterales blood stream infections. Early PDAT may be important not only for antimicrobial stewardship but also for improving patient outcomes.
Conflict of interest statement
Figures

References
-
- Lodise TP, Zhao Q, Fahrbach K, Gillard PJ, Martin A. A systematic review of the association between delayed appropriate therapy and mortality among patients hospitalized with infections due to Klebsiella pneumoniae or Escherichia coli: how long is too long? BMC Infect Dis. 2018;18(1):625. doi: 10.1186/s12879-018-3524-8 - DOI - PMC - PubMed
-
- Kadri SS, Lai YL, Warner S, et al. ; forming the National Institutes of Health Antimicrobial Resistance Outcomes Research Initiative (NIH-ARORI) . Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: a retrospective cohort analysis of prevalence, predictors, and mortality risk in US hospitals. Lancet Infect Dis. 2021;21(2):241-251. doi: 10.1016/S1473-3099(20)30477-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

