Experimental Spin State Determination of Iron(II) Complexes by Hirshfeld Atom Refinement
- PMID: 39714855
- PMCID: PMC11886765
- DOI: 10.1002/chem.202404017
Experimental Spin State Determination of Iron(II) Complexes by Hirshfeld Atom Refinement
Abstract
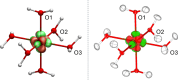
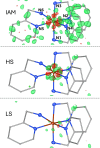
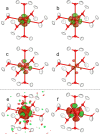
In this study, we present the first experimental determination of the spin state of transition metal complexes by using Hirshfeld Atom Refinement. For the demonstration, the two iron(II) complexes, (NH4)2Fe(SO4)2 ⋅ 6 H2O and lFe(pic)3jCl2 ⋅ EtOH were investigated. The method involves the refinement using wavefunctions of different spin multiplicity and comparison against experimental diffraction data by means of refinement indicators and residual electron density. Our results show a clear distinction between high-spin and low-spin configurations, even for compounds with spin-crossover behavior. The presented work demonstrates the potential of Hirshfeld Atom Refinement for the experimental determination of spin states in transition metal complexes.
Keywords: Hirshfeld Atom Refinement; Iron; Solid-state structures; Spin state determination; X-ray diffraction.
© 2024 The Author(s). Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- M. Besora, J.-L. Carreón-Macedo, Á. Cimas, J. N. Harvey, Spin-State Changes and Reactivity in Transition Metal Chemistry: Reactivity of Iron Tetracarbonyl, in Advances in Inorganic Chemistry, volume 61, pages 573–623, Elsevier 2009.
-
- Meng Y.-S., Liu T., Acc. Chem. Res. 2019, 52, 1369. - PubMed
-
- DeBeer George S., Brant P., Solomon E. I., J. Am. Chem. Soc. 2005, 127, 667. - PubMed
-
- Gawelda W., Cannizzo A., Pham V.-T., Van Mourik F., Bressler C., Chergui M., J. Am. Chem. Soc. 2007, 129, 8199. - PubMed
-
- Vankó G., Glatzel P., Pham V.-T., Abela R., Grolimund D., Borca C. N., Johnson S. L., Milne C. J., Bressler C., Angew. Chem. 2010, 122, 6046. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

