Structural and Functional Mimicry of the Antimicrobial Defensin Plectasin by Analogues with Engineered Backbone Composition
- PMID: 39714882
- PMCID: PMC11875557
- DOI: 10.1002/cbic.202400951
Structural and Functional Mimicry of the Antimicrobial Defensin Plectasin by Analogues with Engineered Backbone Composition
Abstract
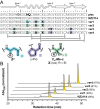
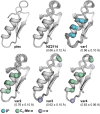
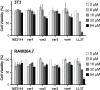
The threat posed by bacteria resistant to common antibiotics creates an urgent need for novel antimicrobials. Non-ribosomal peptide natural products that bind Lipid II, such as vancomycin, represent a promising source for such agents. The fungal defensin plectasin is one of a family of ribosomally produced miniproteins that also exert antimicrobial activity via Lipid II binding. Made up entirely of canonical amino acids, these molecules are potentially more susceptible to degradation by protease enzymes than non-ribosomal counterparts. Here, we report the development of proteomimetic variants of plectasin through the systematic incorporation of artificial backbone connectivity in the domain. An iterative secondary-structure-based design scheme yields a variant with a tertiary fold indistinguishable from the prototype natural product, potent activity against Gram positive bacteria, and low mammalian cell toxicity. Backbone modification is shown to improve oxidative folding efficiency of the disulfide-rich scaffold as well as resistance to proteolytic hydrolysis. These results broaden the scope of design strategies toward protein mimetics as well as folds and biological functions possible in such agents.
Keywords: antimicrobial peptide; disulfide-rich peptide; heterogeneous backbone; plectasin; proteomimetic.
© 2024 The Author(s). ChemBioChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Heterogeneous-Backbone Proteomimetic Analogues of Lasiocepsin, a Disulfide-Rich Antimicrobial Peptide with a Compact Tertiary Fold.ACS Chem Biol. 2022 Apr 15;17(4):987-997. doi: 10.1021/acschembio.2c00138. Epub 2022 Mar 15. ACS Chem Biol. 2022. PMID: 35290019 Free PMC article.
-
Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus.Nature. 2005 Oct 13;437(7061):975-80. doi: 10.1038/nature04051. Nature. 2005. PMID: 16222292
-
Antimicrobial peptide plectasin recombinantly produced in Escherichia coli disintegrates cell walls of gram-positive bacteria, as proven by transmission electron and atomic force microscopy.J Bacteriol. 2025 May 22;207(5):e0045624. doi: 10.1128/jb.00456-24. Epub 2025 Apr 4. J Bacteriol. 2025. PMID: 40183576 Free PMC article.
-
An intimate link between antimicrobial peptide sequence diversity and binding to essential components of bacterial membranes.Biochim Biophys Acta. 2016 May;1858(5):958-70. doi: 10.1016/j.bbamem.2015.10.011. Epub 2015 Oct 21. Biochim Biophys Acta. 2016. PMID: 26498397 Review.
-
Cyclic Peptides as Novel Therapeutic Microbicides: Engineering of Human Defensin Mimetics.Molecules. 2017 Jul 20;22(7):1217. doi: 10.3390/molecules22071217. Molecules. 2017. PMID: 28726740 Free PMC article. Review.
References
-
- MacLean R. C., San Millan A., Science 2019, 365, 1082–1083. - PubMed
-
- CDC, 2019, Antibiotic Resistance Threats in the United States, 2019, U. S. Department of Health and Human Services, DOI 10.15620/cdc:82532. - DOI
-
- CDC, 2022, COVID-19 : U. S. impact on antimicrobial resistance, special report 2022, U. S. Department of Health and Human Services, DOI 10.15620/cdc:117915. - DOI
-
- Miethke M., Pieroni M., Weber T., Brönstrup M., Hammann P., Halby L., Arimondo P. B., Glaser P., Aigle B., Bode H. B., Moreira R., Li Y., Luzhetskyy A., Medema M. H., Pernodet J.-L., Stadler M., Tormo J. R., Genilloud O., Truman A. W., Weissman K. J., Takano E., Sabatini S., Stegmann E., Brötz-Oesterhelt H., Wohlleben W., Seemann M., Empting M., Hirsch A. K. H., Loretz B., Lehr C.-M., Titz A., Herrmann J., Jaeger T., Alt S., Hesterkamp T., Winterhalter M., Schiefer A., Pfarr K., Hoerauf A., Graz H., Graz M., Lindvall M., Ramurthy S., Karlén A., van Dongen M., Petkovic H., Keller A., Peyrane F., Donadio S., Fraisse L., Piddock L. J. V., Gilbert I. H., Moser H. E., Müller R., Nat. Chem. Rev. 2021, 5, 726–749. - PMC - PubMed
-
- Magana M., Pushpanathan M., Santos A. L., Leanse L., Fernandez M., Ioannidis A., Giulianotti M. A., Apidianakis Y., Bradfute S., Ferguson A. L., Cherkasov A., Seleem M. N., Pinilla C., de la Fuente-Nunez C., Lazaridis T., Dai T., Houghten R. A., Hancock R. E. W., Tegos G. P., Lancet Infect. Dis. 2020, 20, e216–e230. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

