Podocyte Injury and Long-Term Kidney Prognosis in Patients with Lupus Nephritis
- PMID: 39714942
- PMCID: PMC12045515
- DOI: 10.34067/KID.0000000688
Podocyte Injury and Long-Term Kidney Prognosis in Patients with Lupus Nephritis
Abstract
Key Points:
Podocyte injury, as indicated by early growth response 1 expression, was correlated with the clinical and histopathological activities of lupus nephritis (LN).
Podocyte injury was associated with poor long-term kidney prognosis in patients with active LN.
To improve the prognosis of patients with LN, treatment strategies on the basis of the degree of podocyte injury may be considered.
Background: Lupus nephritis (LN) is a major complication of SLE. Like other types of GN, podocyte injury has been observed in patients with LN. However, the association between podocyte injury and kidney prognosis in patients with LN has not been well elucidated. The aim of this study was to explore the association between podocyte injury and clinical and histological status and kidney prognosis in patients with LN.
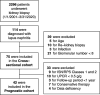
Methods: Seventy-five patients histopathologically diagnosed with LN were enrolled in this study. Early growth response 1 (EGR1) expression in podocytes, representing podocyte injury, was detected through immunohistochemistry. The correlation between the proportion of glomeruli with podocytes expressing EGR1 (%EGR1glo) and the clinical and histological features of LN were evaluated. Subsequently, the association between %EGR1glo and kidney prognosis was examined in a group of patients with LN class 3, 4, or 5 who showed ≥0.5 g/g of urinary protein–creatinine ratio and received immunosuppressive therapy. Hazard ratio was calculated using univariate Cox proportional hazards regression.
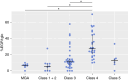
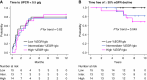
Results: %EGR1glo was highest in patients with LN class 4, significantly correlated with the SLE Disease Activity Index score, urinary protein level, and prevalence of glomeruli showing cellular/fibrocellular crescents, endocapillary hypercellularity, and fibrinoid necrosis and inversely correlated with eGFR. Higher %EGR1glo was significantly associated with sustained ≥30% eGFR decline over 10 years in patients with LN class 3, 4, or 5 (n=42; hazard ratio, 1.58 [95% confidence interval, 1.07 to 2.36] per 10% increase in %EGR1glo). There was no significant interaction between patients grouped by kidney function, urinary protein level, presence of cellular/fibrocellular crescents, degree of tubulointerstitial fibrosis, and LN classification.
Conclusions: Podocyte damage, as indicated by EGR1 expression, was associated with poor long-term kidney prognosis in patients with active LN. Treatment strategies on the basis of the extent of podocyte injury may be necessary.
Keywords: SLE; lupus nephritis; podocyte.
Conflict of interest statement
Disclosure forms, as provided by each author, are available with the online version of the article at
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

