The mitochondrial lactate oxidation complex: endpoint for carbohydrate carbon disposal
- PMID: 39714986
- PMCID: PMC12145959
- DOI: 10.1152/ajpendo.00306.2024
The mitochondrial lactate oxidation complex: endpoint for carbohydrate carbon disposal
Abstract
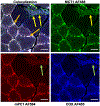
The lactate shuttle concept has revolutionized our understanding and study of metabolism in physiology, biochemistry, intermediary metabolism, nutrition, and medicine. Seminal findings of the mitochondrial lactate oxidation complex (mLOC) elucidated the architectural structure of its components. Here, we report that the mitochondrial pyruvate carrier (mPC) is an additional member of the mLOC in mouse muscle and C2C12 myoblasts and myotubes. Immunoblots, mass spectrometry, and co-immunoprecipitation experiments of mitochondrial preparations revealed abundant amounts of mitochondrial lactate dehydrogenase (mLDH), monocarboxylate transporter (mMCT), basigin (CD147), cytochrome oxidase (COx), and pyruvate carriers 1 and 2 (mPC1 and 2). In addition, using confocal laser scanning microscopy (CLSM) and in situ proximity ligation, we also demonstrated planar and three-dimensional (3-D) colocalization of pyruvate and lactate transporters with COx in fixed mouse skeletal muscle sections and C2C12 myoblasts and myotubes skeletal muscle sections, mouse muscle and C2C12 myoblasts and myotubes myotubes, and C2C12 myoblasts. This work serves as a landmark for configuring the final pathway of carbohydrate oxidation.NEW & NOTEWORTHY We expand on knowledge of the architectural design of the mitochondrial lactate oxidation complex (mLOC); key members are: mitochondrial lactate dehydrogenase (mLDH), monocarboxylate transporter 1 (mMCT1), cytochrome oxidase (COx), basigin scaffolding protein (CD147), and the mitochondrial pyruvate carrier (mPC). The mLOC is key in creating the lower end of the concentration gradient for disposal of lactate and pyruvate.
Keywords: lactate; lactate shuttle; mitochondrial reticulum; pyruvate; skeletal muscle.
Copyright © 2025 The Authors.
Conflict of interest statement
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





Similar articles
-
Colocalization of MCT1, CD147, and LDH in mitochondrial inner membrane of L6 muscle cells: evidence of a mitochondrial lactate oxidation complex.Am J Physiol Endocrinol Metab. 2006 Jun;290(6):E1237-44. doi: 10.1152/ajpendo.00594.2005. Epub 2006 Jan 24. Am J Physiol Endocrinol Metab. 2006. PMID: 16434551
-
Mitochondrial lactate oxidation complex and an adaptive role for lactate production.Med Sci Sports Exerc. 2008 Mar;40(3):486-94. doi: 10.1249/MSS.0b013e31815fcb04. Med Sci Sports Exerc. 2008. PMID: 18379211
-
Effects of lactate administration on mitochondrial enzyme activity and monocarboxylate transporters in mouse skeletal muscle.Physiol Rep. 2019 Sep;7(17):e14224. doi: 10.14814/phy2.14224. Physiol Rep. 2019. PMID: 31512405 Free PMC article.
-
Heart failure-emerging roles for the mitochondrial pyruvate carrier.Cell Death Differ. 2021 Apr;28(4):1149-1158. doi: 10.1038/s41418-020-00729-0. Epub 2021 Jan 20. Cell Death Differ. 2021. PMID: 33473180 Free PMC article. Review.
-
Lactate transport and lactate transporters in skeletal muscle.Can J Appl Physiol. 1997 Dec;22(6):531-52. doi: 10.1139/h97-034. Can J Appl Physiol. 1997. PMID: 9415827 Review.
Cited by
-
Glycolysis to lactylation: Unraveling the metabolic and epigenetic landscape in tissue fibrosis (Review).Mol Med Rep. 2025 Nov;32(5):290. doi: 10.3892/mmr.2025.13655. Epub 2025 Aug 24. Mol Med Rep. 2025. PMID: 40849805 Free PMC article. Review.
References
-
- Pasteur L. Memorie sur la fermentation appelee lactique. In: Annales de Chimie et de Physique, Troisième Série (1st ed.). Paris: Victor Masson, 1858, p. 404–418.
-
- Hill AV, Long CNH, Lupton H. Muscular exercise, lactic acid and the supply and utilisation of oxygen—parts I–III. Proc Roy Soc B 96: 438–475, 1924. doi:10.1098/rspb.1924.0037. - DOI
-
- Meyerhof O. Die energieumwandlungen im muskel III. kohlenhydrat- und milchsaureumsatz im froschmuskel. Pfl00ügers Archiv 185: 11–32, 1920. doi:10.1007/BF01739982. - DOI
-
- Keilin D. The History of Cell Respiration and Cytochrome. Cambridge: Cambridge University Press. 1966, p. 416.
-
- Warburg O The metabolism of carcinoma cells. J Cancer Res 9: 148–163, 1925. doi:10.1158/jcr.1925.148. - DOI

