Study protocol for a randomized controlled trial: Integrating the 'Time-limited Trial' in the emergency department
- PMID: 39715103
- PMCID: PMC11666031
- DOI: 10.1371/journal.pone.0313858
Study protocol for a randomized controlled trial: Integrating the 'Time-limited Trial' in the emergency department
Abstract
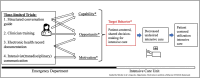
Introduction: Time-limited trial (TLT) is a structured approach between clinicians and seriously ill patients or their surrogates to discuss patients' values and preferences, prognosis, and shared decision-making to use specific therapies for a prespecified period of time in the face of prognostic uncertainty. Some evidence exists that this approach may lead to more patient-centered care in the intensive care unit; however, it has never been evaluated in the emergency department (ED). The study protocol aims to assess the feasibility and acceptability of TLTs initiated in the ED.
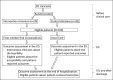
Methods and analysis: We will conduct a parallel group, clinician-level, pilot randomized clinical trial among 40 ED clinicians. We will measure feasibility (e.g., the time it takes to conduct the TLTs by ED clinicians) and clinician and patient-reported acceptability of the TLT, and also track patients' clinical outcomes via medical record review.
Discussion: This study protocol will investigate the potential of TLT initiated in the ED to lead to patient-centered intensive care utilization. By doing so, the study intends to improve palliative care integration for seriously ill older adults in the ED and intensive care unit.
Trial identifier and registry name: ClinicalTrials.gov ID: NCT06378151 https://clinicaltrials.gov/study/NCT06378151; Pre-results; a randomized controlled trial: Time-limited Trials in the Emergency Department.
Copyright: © 2024 Hashimoto et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Dr. Kei Ouchi has received funding personally from Jolly Good, Inc (a virtual reality company) for consulting. This does not alter our adherence to PLOS ONE policies on sharing data and materials. There are no patents, products in development or marketed products associated with this research to declare.
Figures

Similar articles
-
Emergency department-based, nurse-initiated, serious illness conversation intervention for older adults: a protocol for a randomized controlled trial.Trials. 2022 Oct 9;23(1):866. doi: 10.1186/s13063-022-06797-6. Trials. 2022. PMID: 36210436 Free PMC article.
-
Emergency department-initiated palliative care for advanced cancer patients: protocol for a pilot randomized controlled trial.Trials. 2014 Jun 25;15:251. doi: 10.1186/1745-6215-15-251. Trials. 2014. PMID: 24962353 Free PMC article. Clinical Trial.
-
The Chest Pain Choice trial: a pilot randomized trial of a decision aid for patients with chest pain in the emergency department.Trials. 2010 May 17;11:57. doi: 10.1186/1745-6215-11-57. Trials. 2010. PMID: 20478056 Free PMC article. Clinical Trial.
-
Knowledge to action: Rationale and design of the Patient-Centered Care Transitions in Heart Failure (PACT-HF) stepped wedge cluster randomized trial.Am Heart J. 2018 May;199:75-82. doi: 10.1016/j.ahj.2017.12.013. Epub 2017 Dec 27. Am Heart J. 2018. PMID: 29754670 Review.
-
End-of-Life Care, Palliative Care Consultation, and Palliative Care Referral in the Emergency Department: A Systematic Review.J Pain Symptom Manage. 2020 Feb;59(2):372-383.e1. doi: 10.1016/j.jpainsymman.2019.09.020. Epub 2019 Oct 3. J Pain Symptom Manage. 2020. PMID: 31586580

