Nerve regeneration using a Bio 3D conduit derived from umbilical cord-Derived mesenchymal stem cells in a rat sciatic nerve defect model
- PMID: 39715170
- PMCID: PMC11666056
- DOI: 10.1371/journal.pone.0310711
Nerve regeneration using a Bio 3D conduit derived from umbilical cord-Derived mesenchymal stem cells in a rat sciatic nerve defect model
Abstract
Human umbilical cord-derived mesenchymal stromal cells (UC-MSCs), which can be prepared in advance and are presumed to be advantageous for nerve regeneration, have potential as a cell source for Bio 3D conduits. The purpose of this study was to evaluate the nerve regeneration ability of Bio 3D conduits made from UC-MSCs using a rat sciatic nerve defect model.
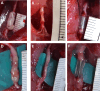
Methods: A Bio 3D conduit was fabricated using a Bio 3D printer by placing UC-MSC spheroids into thin needles according to predesigned 3D data. The conduit was transplanted to bridge the 5-mm gaps of Lewis rat sciatic nerve, and nerve regeneration was evaluated at 8 weeks (Bio 3D group). Transplantation of autologous nerve segments (autograft) and silicone tubes represented the positive and negative control groups, respectively. In a second experiment, immunological reactions were evaluated in Bio 3D, autograft, and allograft groups by histochemical staining of transplanted segments in Brown Norway rats.
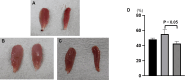
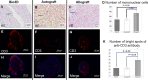
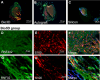
Results: The mean angle of attack value in the kinematic analysis was significantly better in the Bio 3D group (‒20.1 ± 0.5°) than in the silicone group (‒33.7 ± 1.5°) 8 weeks after surgery. The average diameters of myelinated axons were significantly larger in the Bio 3D group (3.61 ± 0.15 μm) than in the silicone group (3.07 ± 0.12 μm), and the number of myelinated axons was significantly higher in the Bio 3D group (11,201 ± 980) than in the silicone group (8117 ± 646). Histological findings (hematoxylin and eosin [HE] staining and anti-CD3 fluorescent immunostaining) showed that rejection was suppressed in the Bio 3D group compared to the allograft group. Based on macroscopic findings and histological findings (anti-human mitochondrial fluorescent immunostaining), UC-MSCs in the Bio 3D conduit disappeared gradually from week 1 to week 8.
Conclusions: The Bio 3D conduit prepared from UC-MSCs was superior to the silicone tube and achieved comparable nerve regeneration to the autologous (autograft) group. Rejection was suppressed in the Bio 3D group compared to the allograft group. Although this study used a xenograft model, we speculate that rejection was low due to the characteristics of UC-MSCs. UC-MSCs are a useful cell source for Bio 3D conduits.
Copyright: © 2024 Iwai et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
There are no patents or marketed products to declare. KN is the co-founder and shareholder of Cyfuse Biomedical K.K., Tokyo, Japan (Cyfuse). YM and SA, who are employees of Cyfuse, contributed to the manufacturing of 3D conduits and Cyfuse provided the bioprinter to manufacture the conduit. The company has the industrial rights related to the bioprinting method used to construct the 3D conduit in this work. Cyfuse provided support in the form of salaries for authors YM, SA and KN and provided research grants to TA, KN and SM. These competing interests do not alter the authors’ adherence to PLOS ONE policies on sharing data and materials.
Figures


Similar articles
-
Efficacy and safety of Bio 3D conduits composed of human umbilical cord-derived mesenchymal stromal cells: A proof-of-concept study in a canine ulnar nerve defect model.Cell Transplant. 2025 Jan-Dec;34:9636897251361711. doi: 10.1177/09636897251361711. Epub 2025 Aug 3. Cell Transplant. 2025. PMID: 40754895 Free PMC article.
-
Bio 3D Conduits Derived from Bone Marrow Stromal Cells Promote Peripheral Nerve Regeneration.Cell Transplant. 2020 Jan-Dec;29:963689720951551. doi: 10.1177/0963689720951551. Cell Transplant. 2020. PMID: 32830545 Free PMC article.
-
The efficacy of a scaffold-free Bio 3D conduit developed from human fibroblasts on peripheral nerve regeneration in a rat sciatic nerve model.PLoS One. 2017 Feb 13;12(2):e0171448. doi: 10.1371/journal.pone.0171448. eCollection 2017. PLoS One. 2017. PMID: 28192527 Free PMC article.
-
Long-Term Outcome of Sciatic Nerve Regeneration Using Bio3D Conduit Fabricated from Human Fibroblasts in a Rat Sciatic Nerve Model.Cell Transplant. 2021 Jan-Dec;30:9636897211021357. doi: 10.1177/09636897211021357. Cell Transplant. 2021. PMID: 34105391 Free PMC article.
-
Nerve regeneration using the Bio 3D nerve conduit fabricated with spheroids.J Artif Organs. 2022 Dec;25(4):289-297. doi: 10.1007/s10047-022-01358-9. Epub 2022 Aug 15. J Artif Organs. 2022. PMID: 35970971 Review.
Cited by
-
Efficacy and safety of Bio 3D conduits composed of human umbilical cord-derived mesenchymal stromal cells: A proof-of-concept study in a canine ulnar nerve defect model.Cell Transplant. 2025 Jan-Dec;34:9636897251361711. doi: 10.1177/09636897251361711. Epub 2025 Aug 3. Cell Transplant. 2025. PMID: 40754895 Free PMC article.
-
Mesenchymal stem cell-based therapy for peripheral nerve injuries: A promise or reality?World J Stem Cells. 2025 Jun 26;17(6):107833. doi: 10.4252/wjsc.v17.i6.107833. World J Stem Cells. 2025. PMID: 40585952 Free PMC article. Review.
-
Optimizing Peripheral Nerve Regeneration: Surgical Techniques, Biomolecular and Regenerative Strategies-A Narrative Review.Int J Mol Sci. 2025 Apr 20;26(8):3895. doi: 10.3390/ijms26083895. Int J Mol Sci. 2025. PMID: 40332790 Free PMC article. Review.
References
-
- Ando M, Ikeguchi R, Aoyama T, Tanaka M, Noguchi T, Miyazaki Y, et al.. Long-Term Outcome of Sciatic Nerve Regeneration Using Bio3D Conduit Fabricated from Human Fibroblasts in a Rat Sciatic Nerve Model. Cell Transplant. 2021. Jan-Dec; 30:9636897211021357. doi: 10.1177/09636897211021357 ; PMCID: PMC8193652. - DOI - PMC - PubMed
-
- Takeuchi H, Sakamoto A, Ikeguchi R, Ohta S, Noguchi T, Ando M, et al.. Muscle Grafts with Doxorubicin Pretreatment Produce "Empty Tubes" in the Basal Laminae, Promote Contentious Maturation of the Regenerated Axons, and Bridge 20-mm Sciatic Nerve Defects in Rats. J Reconstr Microsurg. 2023. Feb;39(2):120–130. doi: 10.1055/s-0042-1750082 Epub 2022 Jul 18. . - DOI - PubMed
-
- Takeuchi H, Ikeguchi R, Noguchi T, Ando M, Yoshimoto K, Sakamoto D, et al.. The efficacy of combining a vascularized biogenic conduit and a decellularized nerve graft in the treatment of peripheral nerve defects: An experimental study using the rat sciatic nerve defect model. Microsurgery. 2022. Mar;42(3):254–264. doi: 10.1002/micr.30853 Epub 2021 Dec 25. . - DOI - PubMed
-
- Yurie H, Ikeguchi R, Aoyama T, Kaizawa Y, Tajino J, Ito A, et al.. The efficacy of a scaffold-free Bio 3D conduit developed from human fibroblasts on peripheral nerve regeneration in a rat sciatic nerve model. PLoS One. 2017. Feb 13;12(2): e0171448. doi: 10.1371/journal.pone.0171448 ; PMCID: PMC5305253. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

