Discovery of Nanosota-EB1 and -EB2 as Novel Nanobody Inhibitors Against Ebola Virus Infection
- PMID: 39715280
- PMCID: PMC11723632
- DOI: 10.1371/journal.ppat.1012817
Discovery of Nanosota-EB1 and -EB2 as Novel Nanobody Inhibitors Against Ebola Virus Infection
Abstract
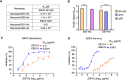
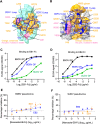
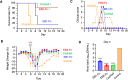
The Ebola filovirus (EBOV) poses a serious threat to global health and national security. Nanobodies, a type of single-domain antibody, have demonstrated promising therapeutic potential. We identified two anti-EBOV nanobodies, Nanosota-EB1 and Nanosota-EB2, which specifically target the EBOV glycoprotein (GP). Cryo-EM and biochemical data revealed that Nanosota-EB1 binds to the glycan cap of GP1, preventing its protease cleavage, while Nanosota-EB2 binds to critical membrane-fusion elements in GP2, stabilizing it in the pre-fusion state. Nanosota-EB2 is a potent neutralizer of EBOV infection in vitro and offers excellent protection in a mouse model of EBOV challenge, while Nanosota-EB1 provides moderate neutralization and protection. Nanosota-EB1 and Nanosota-EB2 are the first nanobodies shown to inhibit authentic EBOV. Combined with our newly developed structure-guided in vitro evolution approach, they lay the foundation for nanobody-based therapies against EBOV and other viruses within the ebolavirus genus.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
B.E.W. and B.S. are co-founders of Turkey Creek Biotechnology. The University of Minnesota has filed patents on Nanosota-EB1 and Nanosota-EB2 with F.L, F.B., and G.Y. as inventors.
Figures



Similar articles
-
Discovery of Nanosota-9 as anti-Omicron nanobody therapeutic candidate.PLoS Pathog. 2024 Nov 26;20(11):e1012726. doi: 10.1371/journal.ppat.1012726. eCollection 2024 Nov. PLoS Pathog. 2024. PMID: 39591462 Free PMC article.
-
Ebola virus-induced eye sequelae: a murine model for evaluating glycoprotein-targeting therapeutics.EBioMedicine. 2024 Jun;104:105170. doi: 10.1016/j.ebiom.2024.105170. Epub 2024 Jun 1. EBioMedicine. 2024. PMID: 38823088 Free PMC article.
-
The Tetherin Antagonism of the Ebola Virus Glycoprotein Requires an Intact Receptor-Binding Domain and Can Be Blocked by GP1-Specific Antibodies.J Virol. 2016 Nov 28;90(24):11075-11086. doi: 10.1128/JVI.01563-16. Print 2016 Dec 15. J Virol. 2016. PMID: 27707924 Free PMC article.
-
The Roles of Ebola Virus Soluble Glycoprotein in Replication, Pathogenesis, and Countermeasure Development.Viruses. 2019 Oct 31;11(11):999. doi: 10.3390/v11110999. Viruses. 2019. PMID: 31683550 Free PMC article. Review.
-
[Research progress on ebola virus glycoprotein].Bing Du Xue Bao. 2013 Mar;29(2):233-7. Bing Du Xue Bao. 2013. PMID: 23757858 Review. Chinese.
Cited by
-
Nanoscale warriors against viral invaders: a comprehensive review of Nanobodies as potential antiviral therapeutics.MAbs. 2025 Dec;17(1):2486390. doi: 10.1080/19420862.2025.2486390. Epub 2025 Apr 9. MAbs. 2025. PMID: 40201976 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

