CAR T-cell therapies for T-cell malignancies: does cellular immunotherapy represent the best chance of cure?
- PMID: 39715467
- PMCID: PMC11876835
- DOI: 10.1182/bloodadvances.2023012263
CAR T-cell therapies for T-cell malignancies: does cellular immunotherapy represent the best chance of cure?
Abstract
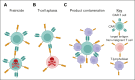
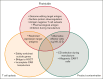
Chimeric antigen receptor T-cell (CAR-T) therapy has proven successful for B-cell lymphomas and leukemias. This success has inspired the development of CAR-T for T-cell malignancies. T-cell lymphomas and T-cell acute lymphoblastic leukemia (T-ALL) are highly heterogenous diseases but are united by poor prognosis in the relapsed/refractory setting and the lack of any novel, targeted therapies. CAR-T therapy is a promising solution for these diseases but carries a number of challenges, principally that target antigens are typically shared between malignant and normal T cells. This can cause issues with fratricide and T-cell aplasia. In this review we discuss the current state of CAR-T treatment for T-ALL and T-cell lymphomas, highlighting recent novel clinical data for T-cell malignancies and discuss lessons that can be learned for future research in this area.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: P.M. owns stock in a publicly traded company, Autolus Ltd. The remaining authors declare no competing financial interests.
Figures
Similar articles
-
Targeting T cell malignancies using CAR-based immunotherapy: challenges and potential solutions.J Hematol Oncol. 2019 Dec 29;12(1):141. doi: 10.1186/s13045-019-0801-y. J Hematol Oncol. 2019. PMID: 31884955 Free PMC article. Review.
-
SOHO State of the Art Updates and Next Questions | CAR T Cells in T Cell Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma.Clin Lymphoma Myeloma Leuk. 2025 Feb;25(2):77-88. doi: 10.1016/j.clml.2024.05.018. Epub 2024 May 31. Clin Lymphoma Myeloma Leuk. 2025. PMID: 38955579 Review.
-
Chimeric antigen receptor T-cell therapy for T-cell acute lymphoblastic leukemia.Haematologica. 2024 Jun 1;109(6):1677-1688. doi: 10.3324/haematol.2023.283848. Haematologica. 2024. PMID: 38832423 Free PMC article. Review.
-
Clinical outcomes and safety of CAR-T cells in treatment of T-Cell acute lymphoblastic leukemia/lymphoma.Ann Hematol. 2025 Jan;104(1):57-63. doi: 10.1007/s00277-024-06132-w. Epub 2024 Dec 18. Ann Hematol. 2025. PMID: 39692783 Review.
-
CAR-T cell therapy in paediatric acute lymphoblastic leukaemia - past, present and future.Br J Haematol. 2020 Nov;191(4):617-626. doi: 10.1111/bjh.17153. Br J Haematol. 2020. PMID: 33190266 Review.
References
-
- Patel AA, Thomas J, Rojek AE, Stock W. Biology and treatment paradigms in T cell acute lymphoblastic leukemia in older adolescents and adults. Curr Treat Options Oncol. 2020;21(7):57. - PubMed
-
- Hogan L.E., Bhatla T., Teachey D.T., et al. Efficacy and safety of daratumumab (DARA) in pediatric and young adult patients (pts) with relapsed/refractory T-cell acute lymphoblastic leukemia (ALL) or lymphoblastic lymphoma (LL): Results from the phase 2 DELPHINUS study. J Clin Oncol. 2022;40(16_suppl) 10001.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

