New Insights from Long-Term Clinical Use of Circulating Tumor DNA-Based Minimal Residual Disease Monitoring in Translocation-Associated Sarcomas
- PMID: 39715595
- PMCID: PMC11974511
- DOI: 10.1159/000543223
New Insights from Long-Term Clinical Use of Circulating Tumor DNA-Based Minimal Residual Disease Monitoring in Translocation-Associated Sarcomas
Abstract
Introduction: Assessment of circulating tumor DNA (ctDNA) as a means to monitor disease activity in translocation-associated tumors has become very popular in clinical practice. However, there are still few studies on its clinical application to date. Our study evaluates the clinical applicability of ctDNA as a biomarker for monitoring minimal residual disease (MRD) in patients with translocation-associated sarcomas.
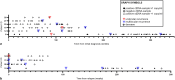
Methods: In this retrospective study, we correlated 285 ctDNA samples from 34 patients diagnosed with translocation-associated sarcoma with the clinical course and images. Blood samples were collected at multiple time points during follow-up (median: 97 weeks, range: 7-398).
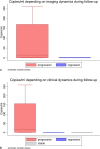
Results: We discovered a significant association between ctDNA levels and the clinical course of the disease, particularly noting differences between patients in remission or with progressive disease (p = 0.001). Furthermore, although we noted that ctDNA levels remained undetectable in a few cases of unilocular recurrence (n = 3), they were consistently higher in patients with multilocular recurrence (n = 14; p = 0.008).
Conclusion: Monitoring ctDNA levels provides highly specific, additional information enabling early recurrence detection in patients with translocation-associated sarcomas during the follow-up and can be integrated into clinical practice. However, MRD monitoring by ctDNA quantification alone does not allow the reliable detection of 100% of unilocular recurrences and should be complemented by the use of conventional imaging techniques.
Introduction: Assessment of circulating tumor DNA (ctDNA) as a means to monitor disease activity in translocation-associated tumors has become very popular in clinical practice. However, there are still few studies on its clinical application to date. Our study evaluates the clinical applicability of ctDNA as a biomarker for monitoring minimal residual disease (MRD) in patients with translocation-associated sarcomas.
Methods: In this retrospective study, we correlated 285 ctDNA samples from 34 patients diagnosed with translocation-associated sarcoma with the clinical course and images. Blood samples were collected at multiple time points during follow-up (median: 97 weeks, range: 7-398).
Results: We discovered a significant association between ctDNA levels and the clinical course of the disease, particularly noting differences between patients in remission or with progressive disease (p = 0.001). Furthermore, although we noted that ctDNA levels remained undetectable in a few cases of unilocular recurrence (n = 3), they were consistently higher in patients with multilocular recurrence (n = 14; p = 0.008).
Conclusion: Monitoring ctDNA levels provides highly specific, additional information enabling early recurrence detection in patients with translocation-associated sarcomas during the follow-up and can be integrated into clinical practice. However, MRD monitoring by ctDNA quantification alone does not allow the reliable detection of 100% of unilocular recurrences and should be complemented by the use of conventional imaging techniques.
Keywords: Cell-free circulating tumor DNA; Liquid biopsy; Minimal residual disease monitoring; Translocation-associated sarcomas.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
S.J., K.K., B.L.-A., and A.T. have no conflicts of interest to declare. M.A.S. has received travel support from ImplanTec, Alphamed, implantcast, and PharmaMar outside of the submitted work. J.S. has received sponsorship and research funds from Eisai, PharmaMar, and Roche and has received payment or other (financial) remuneration from Bayer, Amgen, PharmaMar, and Roche. M.G.S. has received research funding from Amgen and Takeda (paid to institution), consultancy honorary from Pharming, Amgen, and Novartis, and a conference travel grant from CSL Behring. A.E. has received research funding from Qiagen (paid to institution) and received remuneration from Illumina. A.L. reports receiving institutional educational grants by Alphamed, Medacta, and Johnson & Johnson. The funders had no role in the design of this study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Grünewald TGP, Cidre-Aranaz F, Surdez D, Tomazou EM, De Álava E, Kovar H, et al. Ewing sarcoma. Nat Rev Dis Primer. 2018;4(1):5. - PubMed
-
- Krumbholz M, Hellberg J, Steif B, Bäuerle T, Gillmann C, Fritscher T, et al. Genomic EWSR1 fusion sequence as highly sensitive and dynamic plasma tumor marker in ewing sarcoma. Clin Cancer Res. 2016;22(17):4356–65. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

