The molecular mechanisms of cardiac development and related diseases
- PMID: 39715759
- PMCID: PMC11666744
- DOI: 10.1038/s41392-024-02069-8
The molecular mechanisms of cardiac development and related diseases
Abstract
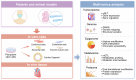
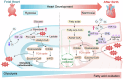
Cardiac development is a complex and intricate process involving numerous molecular signals and pathways. Researchers have explored cardiac development through a long journey, starting with early studies observing morphological changes and progressing to the exploration of molecular mechanisms using various molecular biology methods. Currently, advancements in stem cell technology and sequencing technology, such as the generation of human pluripotent stem cells and cardiac organoids, multi-omics sequencing, and artificial intelligence (AI) technology, have enabled researchers to understand the molecular mechanisms of cardiac development better. Many molecular signals regulate cardiac development, including various growth and transcription factors and signaling pathways, such as WNT signaling, retinoic acid signaling, and Notch signaling pathways. In addition, cilia, the extracellular matrix, epigenetic modifications, and hypoxia conditions also play important roles in cardiac development. These factors play crucial roles at one or even multiple stages of cardiac development. Recent studies have also identified roles for autophagy, metabolic transition, and macrophages in cardiac development. Deficiencies or abnormal expression of these factors can lead to various types of cardiac development abnormalities. Nowadays, congenital heart disease (CHD) management requires lifelong care, primarily involving surgical and pharmacological treatments. Advances in surgical techniques and the development of clinical genetic testing have enabled earlier diagnosis and treatment of CHD. However, these technologies still have significant limitations. The development of new technologies, such as sequencing and AI technologies, will help us better understand the molecular mechanisms of cardiac development and promote earlier prevention and treatment of CHD in the future.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Meilhac, S. M. & Buckingham, M. E. The deployment of cell lineages that form the mammalian heart. Nat. Rev. Cardiol.15, 705–724 (2018). - PubMed
-
- Moorman, A. F. & Christoffels, V. M. Cardiac chamber formation: development, genes, and evolution. Physiol. Rev.83, 1223–1267 (2003). - PubMed
-
- Abu-Issa, R. & Kirby, M. L. Heart field: from mesoderm to heart tube. Annu. Rev. Cell Dev. Biol.23, 45–68 (2007). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

