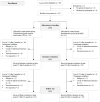
Telemedicine-based exercise intervention in cancer survivors: a non-randomized controlled trial
- PMID: 39715788
- PMCID: PMC11666603
- DOI: 10.1038/s41598-024-83846-x
Telemedicine-based exercise intervention in cancer survivors: a non-randomized controlled trial
Abstract
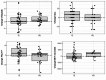
Cancer survivors (CS) often experience treatment-related side effects, such as fatigue, and have reduced physical function. Regular physical activity has been demonstrated to reduce these symptoms and improve cardiopulmonary fitness. Digital solutions are needed to optimize exercise options for CS in aftercare, especially given the significant limitations during the Covid-19 pandemic. This two-armed, non-randomized, controlled intervention study for CS aims to investigate whether a telemedicine-based exercise intervention is as effective as the current standard of care for oncological exercise therapy in aftercare. Patients in the intervention group (n = 61) performed a telemedicine-based exercise program (TE) and patients in the control group (n = 31) participated in an existing rehabilitation sports group (RG) over a six-month intervention period. The primary outcome was cardiopulmonary fitness measured by VO2peak; secondary outcomes included quality of life (QoL), fatigue, and physical activity. A non-inferiority analysis was performed with a predefined non-inferiority margin for relative VO2peak of -1.50 ml/min/kg. Although TE demonstrated a slight advantage in relative VO2peak compared to RG (adjusted mean difference of 0.55 ml/min/kg [95% CI: -2.74; 3.84]), the non-inferiority was not statistically significant. Nevertheless, the implementation of a telemedicine-based exercise intervention indicates that individual patients respond well to this type of exercise program and benefit from the intervention, particularly in terms of QoL. Finding an individualized program for each cancer survivor is the overarching goal. A telemedicine-based exercise intervention may be a promising option, particularly for younger patients.
Keywords: COVID-19; Cancer survivors; Controlled study; Exercise oncology; Supportive cancer care; Wearable activity tracker.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
References
-
- Segal, R. J. et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J. Clin. Oncology: Official J. Am. Soc. Clin. Oncol.27, 344–351. 10.1200/jco.2007.15.4963 (2009). - PubMed
-
- Schmidt, M. E. et al. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: a randomized controlled trial. Int. J. Cancer. 137, 471–480. 10.1002/ijc.29383 (2015). - PubMed
-
- Streckmann, F. et al. Sensorimotor training and whole-body vibration training have the potential to reduce motor and sensory symptoms of chemotherapy-induced peripheral neuropathy-a randomized controlled pilot trial. Support Care cancer. 27, 2471–2478. 10.1007/s00520-018-4531-4 (2019). - PubMed
-
- Kneis, S. et al. It’s never too late - balance and endurance training improves functional performance, quality of life, and alleviates neuropathic symptoms in cancer survivors suffering from chemotherapy-induced peripheral neuropathy: results of a randomized controlled trial. BMC Cancer. 19, 414. 10.1186/s12885-019-5522-7 (2019). - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical