A shorter linker in the bispecific antibody RmAb158-scFv8D3 improves TfR-mediated blood-brain barrier transcytosis in vitro
- PMID: 39715817
- PMCID: PMC11666562
- DOI: 10.1038/s41598-024-83627-6
A shorter linker in the bispecific antibody RmAb158-scFv8D3 improves TfR-mediated blood-brain barrier transcytosis in vitro
Abstract
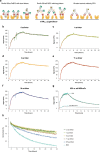
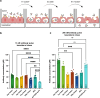
Transferrin Receptor (TfR)-mediated transcytosis across the blood-brain barrier (BBB) enables the uptake of bispecific therapeutic antibodies into the brain. At therapeutically relevant concentrations, bivalent binding to TfR appears to reduce the transcytosis efficiency by receptor crosslinking. In this study, we aimed to improve BBB transcytosis of symmetric antibodies through minimizing their ability to cause TfR crosslinking. We created variants of the previously published RmAb158-scFv8D3, where the linker length between RmAb158 and the mTfR-targeting scFv8D3 was adjusted. We investigated the effect of the linker length on the antibodies' binding kinetics to mTfR using ELISA and LigandTracer assays, and their ability to transcytose across BBB endothelial cells (In-Cell BBB-Trans assay). We show that even a direct fusion without a linker does not alter the antibodies' apparent affinities to mTfR indicating their valency is unlikely affected by the linker length. However, the shortest linker variants demonstrated BBB transcytosis levels comparable to that of the monovalent control at a high antibody concentration and showed an almost two-fold higher level of BBB transcytosis compared to the longer-linker variants at the high concentration. Our new RmAb158-scFv8D3 short-linker variants are examples of symmetric, therapeutic antibodies with improved TfR-binding characteristics to facilitate more efficient brain uptake. We hypothesize that bivalent binding to TfR as such does not negatively affect BBB transcytosis in vitro, but a very short distance between TfR-targeting domains lowers the probability of receptor crosslinking. This study provides valuable insights into antibody-TfR interaction kinetics, contributing to future development of TfR-targeting antibody-based treatments for brain diseases.
Keywords: Bispecific antibodies; Blood-brain-barrier (BBB) shuttle; Monovalent and bivalent binding; Receptor crosslinking; RmAb158-scFv8D3; Transferrin receptor (TfR).
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

