Evaluation of BCRP-Related DDIs Between Methotrexate and Cyclosporin A Using Physiologically Based Pharmacokinetic Modelling
- PMID: 39715910
- PMCID: PMC12011704
- DOI: 10.1007/s40268-024-00495-1
Evaluation of BCRP-Related DDIs Between Methotrexate and Cyclosporin A Using Physiologically Based Pharmacokinetic Modelling
Abstract
Background and objective: This study provides a physiologically based pharmacokinetic (PBPK) model-based analysis of the potential drug-drug interaction (DDI) between cyclosporin A (CsA), a breast cancer resistance protein transporter (BCRP) inhibitor, and methotrexate (MTX), a putative BCRP substrate.
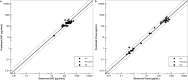
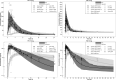
Methods: PBPK models for CsA and MTX were built using open-source tools and published data for both model building and for model verification and validation. The MTX and CsA PBPK models were evaluated for their application in simulating BCRP-related DDIs. A qualification of an introduced empirical uniform in vitro scaling factor of Ki values for transporter inhibition by CsA was conducted by using a previously developed model of rosuvastatin (sensitive index BCRP substrate), and assessing if corresponding DDI ratios were well captured.
Results: Within the simulated DDI scenarios for MTX in the presence of CsA, the developed models could capture the observed changes in PK parameters as changes in the area under the curve ratios (area under the curve during DDI/area under the curve control) of 1.30 versus 1.31 observed and the DDI peak plasma concentration ratios (peak plasma concentration during DDI/peak plasma concentration control) of 1.07 versus 1.28 observed. The originally reported in vitro Ki values of CsA were scaled with the uniform qualified scaling factor for their use in the in vivo DDI simulations to correct for the low intracellular unbound fraction of the CsA effector concentration. The resulting predicted versus observed ratios of peak plasma concentration and area under the curve DDI ratios with MTX were 0.82 and 0.99, respectively, indicating adequate model accuracy and choice of a scaling factor to capture the observed DDI.
Conclusions: All models have been comprehensively documented and made publicly available as tools to support the drug development and clinical research community and further community-driven model development.
© 2024. Galapagos NV and Stephan Schaller, Ingrid Michon, Vanessa Baier, Frederico Severino Martins.
Conflict of interest statement
Declarations. Funding: This study was sponsored by Galapagos NV. Conflicts of Interest/Competing Interests: Stephan Schaller and Vanessa Baier are employees of esqLABS. Frederico Martins was an employee of esqLABS at the time of the work and is now an employee of Simulations Plus, Inc. Ingrid Michon was an employee of SGS Exprimo at the time of the work and is now an employee of Certara. Patrick Nolain was an employee of Galapagos at the time of the work and is now an employee of Novo Nordisk. Amit Taneja was an employee of Galapagos at the time of the work and owns subscription rights in the company, and is now an employee of Simulations Plus, Inc. esqLABS and SGS Exprimo are contract research organisations that were sponsored by Galapagos to conduct the study presented here. Ethics Approval: No ethical approval was required, as all data used in the article have been taken from publicly available sources. Consent to Participate: Not applicable. Consent for Publication: Not applicable. Availability of Data and Material: All available data used are included in the article (either referenced or in the tables). Code Availability: Not applicable. Authors’ Contributions: All authors contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by SS, IM, VB, FM, PN and AT. The first draft of the manuscript was written by SS, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Figures






References
-
- FDA Center for Drug Evaluation and Research. In vitro drug interaction studies: cytochrome P450 enzyme- and transporter-mediated drug interactions guidance for industry. 2020. Available from: https://public4.pagefreezer.com/browse/FDA/15-09-2021T19:20/https:/www.f.... Accessed 18 Oct 2022.
-
- EMA. Investigation of drug interactions. 2018. Available from: https://www.ema.europa.eu/en/investigation-drug-interactions. Accessed 18 Oct 2022.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

