Understanding the National Healthcare Ecosystem to Position Medical Affairs as a Strategic Element: Lessons Learned from AstraZeneca Spain
- PMID: 39715922
- PMCID: PMC11880095
- DOI: 10.1007/s40290-024-00542-x
Understanding the National Healthcare Ecosystem to Position Medical Affairs as a Strategic Element: Lessons Learned from AstraZeneca Spain
Abstract
Introduction: The rapidly evolving healthcare landscape has prompted Medical Affairs (MA) departments within pharmaceutical companies to transition from their traditional role as information providers to becoming strategic partners in the healthcare ecosystem. Responding to the increasing complexity of patient needs and stakeholder dynamics within Spain's national health system, this shift emphasizes the importance of aligning MA functions with broader healthcare goals. Effective transformation requires in-depth assessments of stakeholder trends and expectations. The objectives of this study were to identify key stakeholders in the Spanish healthcare ecosystem, analyze their trends in detail, and evaluate ways in which our MA department should adapt to address them.
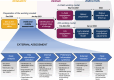
Methods: To support this strategic transformation, we conducted a dual assessment focusing on the Spanish healthcare ecosystem. Using a combination of desk analysis and stakeholder research, we examined external and internal dynamics affecting the MA department's role. This approach identified the perspectives and needs of key stakeholders across three main communities: patients, healthcare professionals, and institutional bodies, offering insights into stakeholder-specific expectations and broader industry macrotrends.
Results: The identification of 16 pivotal stakeholders in Spain's healthcare ecosystem underscores the complex array of needs and expectations that shape MA's evolving role. For the patient community, key trends include the demand for accessible information, emotional support resources, and tools to enhance treatment adherence and chronic disease management. Among clinicians and key external experts, there is an increasing need for current medical resources, digital integration in care processes, and collaborative research opportunities. Institutional stakeholders emphasize sustainable integration of therapeutic innovations, budget predictability, and public-private collaboration to enhance healthcare quality and access. Beyond these specific needs, transversal trends affecting all stakeholders were identified, including the acceleration of medical innovation, demand for value-added services, and the expansion of digital tools and data-driven decision making. Macrotrends within the pharmaceutical industry - such as patient-centric approaches, the growth of digital health, and data-focused business models - further shape the industry's response to evolving healthcare challenges. A unified organizational vision, reflected in shared objectives and priorities, is crucial for cohesive strategy implementation.
Conclusion: This research underscores the essential role of MA departments in redefining their influence within healthcare systems. By aligning MA activities with stakeholder trends, pharmaceutical companies can reposition MA as a central pillar within the healthcare ecosystem. Our assessment of the Spanish health system provides a strategic framework that can be easily implemented by other MA departments in the industry to adapt to evolving stakeholder dynamics worldwide.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Funding: No funding was received to conduct the study analysis nor the preparation of the manuscript, with the exception of medical writing support, which was funded by AstraZeneca. The open access fee was also funded by AstraZeneca. Conflicts of Interest: Ana Pérez Dominguez, Lucía Regadera Anechina, Jorge Marinich, and Inmaculada Iglesias declare being employed and owning stock of AstraZeneca. Marc Cortés has no conflicts of interest to declare in relation to this sudy. Availability of Data and Material: All data generated during this study are included in this published article. Author Contributions: All authors contributed to the conception and design of the study, as well as the investigation and analysis performed. The first draft of the manuscript was written by Ana Pérez Dominguez. All authors critically reviewed, edited, and commented on the manuscript until the final version was obtained. The final version of the manuscript was read and approved by all authors. Ethics Approval: Not applicable. Consent to Participate: Not applicable. Consent for Publication: Not applicable. Code Availability: Not applicable.
Figures
References
-
- MAPS Visionary Working Group. The Future of Medical Affairs 2030. Medical Affairs Professional Society [Internet] 2022; Available from: https://medicalaffairs.org/futuremedical-affairs-2030/. Accessed 12 Nov 2024
-
- Algazy J, Garcia A, S. R, Westra A, Zemp A. A vision for medical affairs 2030: Five priorities for patient impact. McKinsey & Company [Internet] 2023; Available from: https://www.mckinsey.com/industries/life-sciences/our-insights/a-vision-.../. Accessed 12 Nov 2024
MeSH terms
LinkOut - more resources
Full Text Sources
Medical