Meta-analysis of the efficacy and safety of vamorolone in Duchenne muscular dystrophy
- PMID: 39715964
- PMCID: PMC12003609
- DOI: 10.1007/s10072-024-07939-1
Meta-analysis of the efficacy and safety of vamorolone in Duchenne muscular dystrophy
Abstract
Background: Duchenne muscular dystrophy (DMD) is a severe neuromuscular disorder, often leading to wheelchair dependence by age 13 with limited treatment options, largely relying on glucocorticosteroids. We assessed the efficacy and safety of vamorolone, a modified synthetic corticosteroid, for DMD.
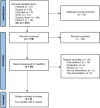
Methods: We performed a systematic review and meta-analysis using seven databases including prospective studies comparing vamorolone with glucocorticosteroids or placebo in DMD patients. We extracted data on efficacy and safety outcomes. We built fixed effects models to assess mean differences. (PROSPERO: CRD42023396908).
Results: Out of 210 identified records, two study reports were included in meta-analysis providing data from 210 patients. Vamorolone at 2.0 mg/kg/day was associated with improvement time to climb four stairs velocity (MD = 0.05 95% CI [0.03 to 0.08] P = 0.0002), and time stand from supine velocity (MD = 0.07 95% CI [0.01 to 0.07] P = 0.007). A higher dose of 6.0 mg/kg/day was additionally associated with higher time to run/walk 10 m velocity (MD = 0.10 95% CI [-0.0.1 to 0.21] P = 0.07, I2 = 0%). Among these beneficial effects only improvement in time to climb four stairs velocity was sustained after a follow-up period of 30 months. Vamorolone did not inhibit growth but increased the risk of weight gain, suppression of adrenal function, and insulin resistance.
Conclusion: The results of our systematic review and meta-analyis are suggestive of improved efficacy and safety of vamorolone for DMD compared to standard glucocorticosteroids but the external validity of these findings as well as the medication's long-term effects remain to be determined.
Keywords: 6MWT; Drug; Muscle; NSAA; Steroid; TTCLIMB; TTRW; TTSTAND; Vamorolone.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and Informed consent: Consent from the ethics committee was not required for the research. Informed consent was not applicable for this systematic review and meta-analysis. Informed consent: Not applicable. Conflicts of interest: The authors declare no conflict of interest.
Figures


References
-
- Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D, Case LE, Clemens PR, Hadjiyannakis S, Pandya S, Street N, Tomezsko J, Wagner KR, Ward LM, Weber DR, DMD Care Considerations Working Group (2018). Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol 17(3):251–267. 10.1016/S1474-4422(18)30024-3 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

