Geranylgeranyl Pyrophosphate Promotes Profibrotic Factors and Collagen-Specific Chaperone HSP47 in Fibroblasts
- PMID: 39716037
- PMCID: PMC11666423
- DOI: 10.1111/jcmm.70273
Geranylgeranyl Pyrophosphate Promotes Profibrotic Factors and Collagen-Specific Chaperone HSP47 in Fibroblasts
Abstract
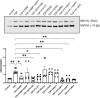
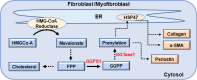
Fibrosis, characterised by excessive extracellular matrix deposition, contributes to both organ failure and significant mortality worldwide. Whereas fibroblasts are activated into myofibroblasts, marked by phenotypic factors such as α-smooth muscle actin (α-SMA), periostin, fibroblast activation protein (FAP) and heat shock protein 47 (HSP47), the cellular processes of trans-differentiation for fibrosis development remain poorly understood. Herein, we hypothesised that the molecular signalling of geranylgeranyl pyrophosphate (GGPP), a crucial biochemical molecule for protein prenylation, is essential in the regulation of profibrotic mechanisms for fibroblast-to-myofibroblast activation. To test this hypothesis, we demonstrated pharmacological inhibition of geranylgeranyl pyrophosphate synthase (GGPS1) significantly decreased TGF-β1-dependent myofibroblast differentiation assessed by reduced α-SMA, periostin, FAP and HSP47 expression. Exogenous GGPP in the presence of GGPS1 inhibition restored TGF-β1-induced differentiation, supporting posttranslational requirements of GGPP modification during myofibroblast differentiation. Selective inhibition of either geranylgeranyl transferase or farnesyl transferase significantly impacted TGF-β1-induced myofibroblast α-SMA and HSP47 expression. The importance of protein prenylation as a key regulator of myofibroblast differentiation was remarkably revealed by an unexpected decrease in HSP47 expression. In contrast, direct HSP47 inhibition not only suppressed TGF-β1-induced α-SMA expression but surprisingly could not be rescued using exogenous GGPP. A selective role for the ER-resident chaperone HSP47 expression downstream of GGPP was suggested when the effects of GGPS1 inhibition on periostin expression were counteracted by GGPP and geranylgeranyl transferase inhibition. Taken together, our findings underscore for the first time the functional role of cholesterol synthesis-independent GGPP-dependent pathway in fibroblast-to-myofibroblast transition and open new potential therapeutic targets for antifibrosis therapies.
Keywords: HSP47; differentiation; fibroblasts; geranylgeranyl pyrophosphate.
© 2024 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

