Identifying the presence of atrial fibrillation during sinus rhythm using a dual-input mixed neural network with ECG coloring technology
- PMID: 39716064
- PMCID: PMC11665121
- DOI: 10.1186/s12874-024-02421-0
Identifying the presence of atrial fibrillation during sinus rhythm using a dual-input mixed neural network with ECG coloring technology
Abstract
Background: Undetected atrial fibrillation (AF) poses a significant risk of stroke and cardiovascular mortality. However, diagnosing AF in real-time can be challenging as the arrhythmia is often not captured instantly. To address this issue, a deep-learning model was developed to diagnose AF even during periods of arrhythmia-free windows.
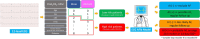
Methods: The proposed method introduces a novel approach that integrates clinical data and electrocardiograms (ECGs) using a colorization technique. This technique recolors ECG images based on patients' demographic information while preserving their original characteristics and incorporating color correlations from statistical data features. Our primary objective is to enhance atrial fibrillation (AF) detection by fusing ECG images with demographic data for colorization. To ensure the reliability of our dataset for training, validation, and testing, we rigorously maintained separation to prevent cross-contamination among these sets. We designed a Dual-input Mixed Neural Network (DMNN) that effectively handles different types of inputs, including demographic and image data, leveraging their mixed characteristics to optimize prediction performance. Unlike previous approaches, this method introduces demographic data through color transformation within ECG images, enriching the diversity of features for improved learning outcomes.
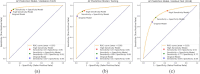
Results: The proposed approach yielded promising results on the independent test set, achieving an impressive AUC of 83.4%. This outperformed the AUC of 75.8% obtained when using only the original signal values as input for the CNN. The evaluation of performance improvement revealed significant enhancements, including a 7.6% increase in AUC, an 11.3% boost in accuracy, a 9.4% improvement in sensitivity, an 11.6% enhancement in specificity, and a substantial 25.1% increase in the F1 score. Notably, AI diagnosis of AF was associated with future cardiovascular mortality. For clinical application, over a median follow-up of 71.6 ± 29.1 months, high-risk AI-predicted AF patients exhibited significantly higher cardiovascular mortality (AF vs. non-AF; 47 [18.7%] vs. 34 [4.8%]) and all-cause mortality (176 [52.9%] vs. 216 [26.3%]) compared to non-AF patients. In the low-risk group, AI-predicted AF patients showed slightly elevated cardiovascular (7 [0.7%] vs. 1 [0.3%]) and all-cause mortality (103 [9.0%] vs. 26 [6.4%]) than AI-predicted non-AF patients during six-year follow-up. These findings underscore the potential clinical utility of the AI model in predicting AF-related outcomes.
Conclusions: This study introduces an ECG colorization approach to enhance atrial fibrillation (AF) detection using deep learning and demographic data, improving performance compared to ECG-only methods. This method is effective in identifying high-risk and low-risk populations, providing valuable features for future AF research and clinical applications, as well as benefiting ECG-based classification studies.
Keywords: Atrial fibrillation; Deep learning; Demographic information; Dual-input mixed neural network; ECG colorization; Sinus rhythm.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Institutional Review Board (2017–10-009BC) at Taipei Veterans General Hospital, Taipei, Taiwan. All methods were carried out following the regulations of the Institutional Review Board. The Internal Review Board of Taipei Veterans General Hospital granted an exemption from the need to secure informed consent due to the thorough de-identification of patient data. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures






Similar articles
-
Prediction of incident atrial fibrillation using deep learning, clinical models, and polygenic scores.Eur Heart J. 2024 Dec 7;45(46):4920-4934. doi: 10.1093/eurheartj/ehae595. Eur Heart J. 2024. PMID: 39217446 Free PMC article.
-
Identification of Atrial Fibrillation With Single-Lead Mobile ECG During Normal Sinus Rhythm Using Deep Learning.J Korean Med Sci. 2024 Feb 5;39(5):e56. doi: 10.3346/jkms.2024.39.e56. J Korean Med Sci. 2024. PMID: 38317452 Free PMC article.
-
An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction.Lancet. 2019 Sep 7;394(10201):861-867. doi: 10.1016/S0140-6736(19)31721-0. Epub 2019 Aug 1. Lancet. 2019. PMID: 31378392
-
Review of Deep Learning-Based Atrial Fibrillation Detection Studies.Int J Environ Res Public Health. 2021 Oct 28;18(21):11302. doi: 10.3390/ijerph182111302. Int J Environ Res Public Health. 2021. PMID: 34769819 Free PMC article. Review.
-
Artificial intelligence and atrial fibrillation.J Cardiovasc Electrophysiol. 2022 Aug;33(8):1932-1943. doi: 10.1111/jce.15440. Epub 2022 Mar 15. J Cardiovasc Electrophysiol. 2022. PMID: 35258136 Free PMC article. Review.
References
-
- Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ, et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet. 2019;394(10201):861–7. 10.1016/S0140-6736(19)31721-0. - PubMed
-
- Liaqat S, Dashtipour K, Zahid A, Assaleh K, Arshad K, Ramzan N. Detection of atrial fibrillation using a machine learning approach. Information. 2020;11(12):549. 10.3390/info11120549.
-
- Noseworthy PA, Attia ZI, Behnken EM, Giblon RE, Bews KA, Liu S, et al. Artificial intelligence-guided screening for atrial fibrillation using electrocardiogram during sinus rhythm: a prospective non-randomised interventional trial. Lancet. 2022;400(10359):1206–12. 10.1016/S0140-6736(22)01637-3. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

