Non-linear effects of age in reporting of adverse events following influenza immunization in Zhejiang, China
- PMID: 39716104
- PMCID: PMC11664878
- DOI: 10.1186/s12879-024-10385-1
Non-linear effects of age in reporting of adverse events following influenza immunization in Zhejiang, China
Abstract
Background: Previous evidence had suggested age and sex affect the reporting rate of adverse events following immunization (AEFI), but with little exploration of potential their non-linear and interaction effects on AEFIs. Examining these non-linear effects could be beneficial for identifying high-risk populations.
Methods: Using AEFI records and vaccination data from national passive surveillance system of adverse event following immunization and Zhejiang provincial immunization information system in the 2021-2022 influenza season, respectively. The effects of age and sex on AEFIs were analyzed through the generalized additive model (logistic regression with a smooth term) to estimate non-linear characteristics after adjusting for other co-variables (adopted significance level p < 0.05).
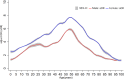
Results: There were 1,259,975 influenza vaccine doses administered and 1304 AEFI records reported during the 2021-2022 influenza season, with a reporting rate of 10.35/10,000 doses. The odds of reporting an AEFI increased from 6 months of age, peaking at about 54 years of age, then gradually declined. The odds of females experiencing AEFIs are higher than that of males. The data model indicated clear effects of age, sex, and their interaction (p < 0.01) on reporting rate of AEFI. Concomitant vaccination and vaccine type were also the impact factors for reporting rate of AEFI.
Conclusion: This study revealed a non-linear property in age and the AEFI odds, with a significant interaction and higher reporting rate in females. In addition, the odds of AEFI increased with co-administration compared to separate vaccination.
Keywords: Adverse events following immunization; Age; Influenza vaccine; Interactions; Non-linear effects.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Institutional review board statement: The study was approved by the Institutional Ethics Committees of Zhejiang provincial center for disease control and prevention (TR-027). No personal information, including the names of specific participants involved in the monitoring, will be disclosed. Written informed consent from the participants or legal guardians were not required for participation in this study according to national legislation and institutional requirements [29]. Informed consent: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Crawford NW, Clothier H, Hodgson K, Selvaraj G, Easton ML, Buttery JP. Active surveillance for adverse events following immunization. Expert Rev Vaccines. 2014;13(2):265–76. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical