Effectiveness and feasibility of a theory-informed intervention to improve Mediterranean diet adherence, physical activity and cognition in older adults at risk of dementia: the MedEx-UK randomised controlled trial
- PMID: 39716203
- PMCID: PMC11667912
- DOI: 10.1186/s12916-024-03815-z
Effectiveness and feasibility of a theory-informed intervention to improve Mediterranean diet adherence, physical activity and cognition in older adults at risk of dementia: the MedEx-UK randomised controlled trial
Abstract
Background: Despite an urgent need for multi-domain lifestyle interventions to reduce dementia risk, there is a lack of interventions which are informed by theory- and evidence-based behaviour change strategies, and no interventions in this domain have investigated the feasibility or effectiveness of behaviour change maintenance. We tested the feasibility, acceptability and cognitive effects of a personalised theory-based 24-week intervention to improve Mediterranean diet (MD) adherence alone, or in combination with physical activity (PA), in older-adults at risk of dementia, defined using a cardiovascular risk score.
Methods: Participants (n = 104, 74% female, 57-76 years) were randomised to three parallel intervention arms: (1) control, (2) MD, or (3) MD + PA for 24 weeks and invited to an optional 24-week follow-up period with no active intervention. Behaviour change was supported using personalised targets, a web-based intervention, group sessions and food provision. The primary outcome was behaviour change (MD adherence and PA levels), and the secondary outcomes included feasibility and acceptability, cognitive function, cardiometabolic health (BMI and 24-h ambulatory blood pressure) and process measures.
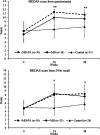
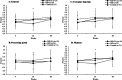
Results: The intervention was feasible and acceptable with the intended number of participants completing the study. Participant engagement with group sessions and food provision components was high. There was improved MD adherence in the two MD groups compared with control at 24 weeks (3.7 points on a 14-point scale (95% CI 2.9, 4.5) and 48 weeks (2.7 points (95% CI 1.6, 3.7)). The intervention did not significantly change objectively measured PA. Improvements in general cognition (0.22 (95% CI 0.05, 0.35), memory (0.31 (95% CI 0.10, 0.51) and select cardiovascular outcomes captured as underpinning physiological mechanisms were observed in the MD groups at 24 weeks.
Conclusions: The intervention was successful in initiating and maintaining dietary behaviour change for up to 12 months which resulted in cognitive benefits. It provides a framework for future complex behaviour change interventions with a range of health and well-being endpoints.
Trial registration: ClinicalTrials.gov NCT03673722.
Keywords: Behaviour change; Dementia; Mediterranean diet; Physical activity; RCT.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical approval for the study was given by the National Research Ethics Committee Northern Ireland (18/NI/0191). Participants provided initial consent for the 24-week study intervention during the online screening and written consent during the in-person screening. During the initial 24-week intervention, participants were invited to take part in the 24- to 48-week behaviour maintenance phase, and further written consent was obtained. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Livingston G, Huntley J, Liu KY, Costafreda SG, Selbæk G, Alladi S, Ames D, Banerjee S, Burns A, Brayne C, et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. The Lancet. 2024;404:572–628. - PubMed
-
- Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, Backman L, Hanninen T, Jula A, Laatikainen T, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–63. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

