Piezo1 overexpression in the uterus contributes to myometrium contraction and inflammation-associated preterm birth
- PMID: 39716206
- PMCID: PMC11667994
- DOI: 10.1186/s12967-024-05978-y
Piezo1 overexpression in the uterus contributes to myometrium contraction and inflammation-associated preterm birth
Abstract
Background: Preterm birth, a leading cause of perinatal mortality and morbidity, is often associated with inflammation and aberrant myometrial contractions. This study investigates the role of Piezo1, a mechanosensitive ion channel, in myometrium contraction and inflammation-associated preterm birth.
Methods: We employed Western blotting, Immunofluorescence, and Quantitative real-time PCR techniques to examine Piezo1 expression in uterine tissues. Functional assays, including myometrial contractility studies and cell contraction assays, were conducted to elucidate the effects of Piezo1 on myometrial contractions. Piezo1 inhibitors and gene knockdown techniques were used to investigate the impact of Piezo1 on inflammation-associated preterm birth, complemented by inflammatory cytokine profiling and calcium imaging to investigate the mechanism.
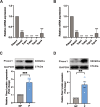
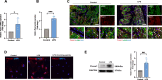
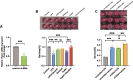
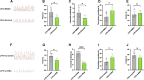
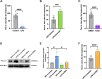
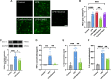
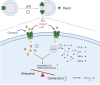
Results: Our findings reveal that Piezo1 is the predominant mechanosensitive channel in mouse myometrium tissue and mouse primary uterine smooth muscle (pUSMCs), with increased expression during mouse and human pregnancy. Following lipopolysaccharide (LPS) intrauterine injection, Piezo1 mRNA and protein levels were elevated in the mouse uterine smooth muscle layer. Direct pharmacologic activation of Piezo1 by Yoda1 increased the contraction of pUSMCs and shortened the pregnancy duration. In contrast, inhibition with Gsmtx4 or siRNA knockdown of Piezo1 attenuated LPS-induced pUSMCs contraction and spontaneous uterine myometrium contraction. Additionally, blocking or knocking down Piezo1 prolonged the pregnancy in an LPS-induced preterm birth model. Yoda1 stimulation increased intracellular Ca2+ levels in pUSMCs, while Gsmtx4 reduced these levels. Gsmtx4 decreased cox-2 expression and inflammation factors in LPS-stimulated pUSMCs.
Conclusions: These results suggest that Piezo1 acts as a critical regulator of uterine function, and its overexpression may predispose to preterm labor through heightened myometrial activity and inflammation. The study underscores the potential of targeting Piezo1 as a therapeutic strategy to mitigate preterm birth associated with uterine inflammation.
Keywords: Mechanosensitive ion channels; Myometrium contraction; Piezo1; Preterm birth.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Animal Ethics Committee of West China Second University Hospital of Sichuan University (NO. 2023015), and all animal procedures were performed following the National Institutes of Health guidelines for the care and use of laboratory animals. The human tissues obtained were approved by the institutional research ethics committee of West China Second University Hospital (No. 2024140). Consent for publication: Not applicable. Competing interests: All authors declare that there are no conflicts of interest.
Figures
Similar articles
-
Novel identification and modulation of the mechanosensitive Piezo1 channel in human myometrium.J Physiol. 2023 May;601(9):1675-1690. doi: 10.1113/JP283299. Epub 2022 Aug 8. J Physiol. 2023. PMID: 35941750 Free PMC article.
-
RAC1 is involved in uterine myometrium contraction in the inflammation-associated preterm birth.Reproduction. 2022 Sep 20;164(4):169-181. doi: 10.1530/REP-21-0186. Print 2022 Oct 1. Reproduction. 2022. PMID: 36018772 Free PMC article.
-
Inflammation-induced PFKFB3-mediated glycolysis promoting myometrium contraction through the PI3K-Akt-mTOR pathway in preterm birth mice.Am J Physiol Cell Physiol. 2025 Mar 1;328(3):C895-C907. doi: 10.1152/ajpcell.00704.2024. Epub 2025 Feb 5. Am J Physiol Cell Physiol. 2025. PMID: 39907705
-
Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour.Eur J Obstet Gynecol Reprod Biol. 2009 May;144 Suppl 1:S2-10. doi: 10.1016/j.ejogrb.2009.02.044. Epub 2009 Mar 18. Eur J Obstet Gynecol Reprod Biol. 2009. PMID: 19299064 Review.
-
Progesterone control of myometrial contractility.J Steroid Biochem Mol Biol. 2023 Nov;234:106397. doi: 10.1016/j.jsbmb.2023.106397. Epub 2023 Sep 6. J Steroid Biochem Mol Biol. 2023. PMID: 37683774 Review.
References
-
- Rubens CE, Sadovsky Y, Muglia L, Gravett MG, Lackritz E, Gravett C. Prevention of preterm birth: harnessing science to address the global epidemic. Sci Transl Med. 2014;6(262):262sr5. - PubMed
-
- Walani SR. Global burden of preterm birth. Int J Gynaecol Obstet. 2020;150(1):31–3. - PubMed
-
- Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;362(6):529–35. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

